Home / Journal / Endocrinology, Diabetes and Obesity
Proposed Person-Specific Treatment Pathway for Cardiovascular Metabolic Renal Disease & Implications to Population Health
Value-based care Implementation science Treatment pathway Optimized Quintet Multi-interdisciplinary team Population health Cardiovascular metabolic renal disease Target therapies Continuation therapy T2D and CKD Chronic Kidney Disease T2D and PAD Peripheral arterial disease Heart failure T2D and heart failure Ischemic disease Diabetes HbA1c Medication adherence
Michael Attanasio, Megan Santanna
DOI: 10.31532/EndocrinolDiabetesObes.2.1.002 24 Sep 2021
Abstract
The WHO considered Diabetes a global epidemic in its first Global Report on Diabetes published in 2016.1 Diabetic treatment standards of care have shifted immensely in the previous decade with the emergence of new therapeutic classes and important evidence from Cardiovascular and Renal Outcomes Trials (CVOT). These studies have helped to draw connections that newer therapies are not simply reducing hyperglycemia but lessen risk of atherosclerotic events, heart failure and worsening renal function. Through this meaningful research, there is a growing body of evidence to begin approaching the treatment of diabetes as a cardiovascular-metabolic-renal disease. There is much work to be completed in this area as there is a notable content gap that directly tackles both the complexities of disease and comprehensive therapeutic treatment implementation in diabetes patients. Data overload and rapid changes to standards of care have left providers, payers, and PBM’s with unclear direction forward. This paper works to highlight the importance of the previous decade of research including the CVOT outcomes, illustrates an optimized person-specific pathway for therapeutic treatment of the cardiovascular metabolic renal disease patient and assesses the implications to population health including care delivery and total cost of care. Our proposed pathway joins guideline-based treatment and CVOT data to provide a practical application to implement in incremental patient care. Finally, provides a real-world framework implementation plan of a multi-interdisciplinary team for a value-based outcome driven initiative.
Keywords
Value-based care, implementation science, treatment pathway, Optimized Quintet, multi- interdisciplinary team, population health, cardiovascular metabolic renal disease, target therapies, continuation therapy, T2D and CKD, Chronic Kidney Disease, T2D and PAD, peripheral arterial disease, heart failure, T2D and heart failure, Ischemic disease, diabetes HbA1c, medication adherence
Diabetes is a Growing Problem
Diabetics do not live in a compartmentalized disease state. The complexity of diabetes extends and impacts nearly every organ system in the human body as demonstrated in the Ominous Octet published over a decade ago.2 Diabetic care is expanding beyond the treatment of hyperglycemia to include early identification, evaluation and treatment of complications and comorbidities. The focus of this article concentrates on Coronary Artery Disease, Cerebral Vascular Disease, Chronic Kidney Disease (CKD), Peripheral Arterial Disease (PAD), and Heart Failure.
This paper concentrates on the prevalence of diabetic comorbidities and complications to demonstrate the need for a therapeutic pathway devised through an understanding of FDA indicated treatments. This is not meant to be an exhaustive review of all diabetic therapy trials. We aim to progress past the well-established standard of care and progress from intermediary measures to outcomes-based treatment pathways. The impact of the proposed treatment pathway provides future opportunities for improvement in population health.
Epidemiology of Macro & Microvascular Complications & Comorbidities Cerebrovascular Accident (CVA) Prevalence in T2D
Cerebrovascular accidents are a significant and well-recognized risk factor of T2D patients. Approximately one third of all stroke patients have a diabetic diagnosis.3 Diabetics are roughly 1.5 times more likely to have a stroke compared to non-diabetics.4 Hyperglycemia increases the risk of stroke in diabetic patients. In addition, blood glucose levels at initial onset of stroke are highly correlated with stroke outcomes .5 Non-fatal strokes are devastating to patient quality of life and also increases the risk of a recurrent stroke. About 15% of T2D experience a recurrent stroke within two years of their initial CVA. 6 Recurrent strokes lead to increased complications and poorer patient outcomes.
Heart Failure Prevalence in T2D
Diabetics are two to four times more likely to develop heart failure. 7 It is estimated that 25% of diabetic patients also have a chronic heart failure diagnosis and upwards of 40% are diagnosed with acute heart failure. Several studies have attempted to understand the occurrence of hospitalizations in patients diagnosed with diabetes and heart failure. One study found that these patients had a two-fold incidence of hospitalization compared to non-diabetic heart-failure patients. 8 Not only does diabetes increase the occurrence of hospitalizations but it also lengthened the hospital stay in diabetics with heart failure.8 Another multi-national study enrolled over 45,000 patients in a 4-year follow up program comparing outcomes of diabetic patients with established co-morbidities (19699) and patients without diabetes.9 After the 4 year follow up, researchers found that overall patients with diabetes had an increased rate for CV death, MI and stroke compared to the non-diabetic cohorts.9 The study also calculated the probabilities of diabetics with heart failure being hospitalized and found that these patients had a 33% increase in the odds of hospitalization vs non-diabetic heart failure patients.
Cardiovascular Disease in T2D
Cardiovascular Disease is the leading cause of death in diabetic patients (National Diabetes Educational Program, 2011). 68% of T2D experience a CV (CVA, CAD, PAD) death.10 The International Diabetes Federation estimates diabetes reduces life expectancies by 10 years with the largest contributor being cardiovascular disease.11,12 Over a 7-year period of monitoring patients with T2D, death rates for patients with a previous history of MI were 42% compared to 15.4% of those with no history of MI.12 A 2018 study published in the journal of Cardiovascular Diabetology reviewed multiple previous studies with 4,549,481 patients and found that cardiovascular disease and T2D was present in 32.3% of the patients.12 Half (50.3%) of deaths observed in this study were attributed to CVD and T2D.12
Peripheral Arterial Disease (PAD) Prevalence in T2D
Presence of Peripheral Arterial Disease in diabetics is another large contributor to adverse health outcomes and is a common comorbidity of this population. Epidemiological estimates suggest nearly 20% of diabetics below 40 years of age have PAD as a diagnosis and that number increased to 29% as diabetics age over 50.13 Amputation is a frequent complication of diabetics with PAD, in fact, 60% of all non-traumatic amputations occurring in the US are on diabetic patients.14 Nearly 1 in 10 diabetics have a foot ulcer.15 25% of ulcers that will not heal require amputation.16 Approximately 20% of all diabetic hospitalizations are due to foot ulcers which can be attributed to both PAD and diabetic neuropathy.17 Research suggests that amputations in diabetics with PAD are increasing.18
Chronic Kidney Disease (CKD) Prevalence in T2D
35% of diabetics in the US over the age of 20 are diagnosed with Chronic Kidney Disease.19 CKD has been referred to as a “disease multiplier” often occurring with other complication of which hypertension and diabetes are the primary sources.19 CKD progression to End Stage Renal Disease (ESRD) is significantly impacted by poor glycemic control and uncontrolled hypertension. 75% of kidney failure is a result of diabetes and hypertension.20 In 2014, 44% of patients starting hemodialysis for ERSD listed diabetes as the primary cause.21 More than half of all deaths in patients with ESRD are attributable to CVD (NIDDKD, 2016). Patients with ESRD have average 1.7 admissions per year. 19 The cost of care for Medicare patients on hemodialysis is extraordinary, averaging around $90,000 annually.22 Comparatively, the average cost per Medicare beneficiary in 2018 was $11,172.23 ESRD patients account for 7% of the Medicare budget.22
The link between Diabetes and Cardiovascular disease is evident in the high proportion of patients who develop macro and microvascular disease. Complications such as Coronary Artery Disease, Heart Failure, Peripheral Artery Disease and Chronic Kidney Disease are disproportionately represented in the diabetic population. Further, patients who develop complications such as chronic kidney disease are at higher risk of worsening morbidity and mortality from emergence or worsening of additional cardiovascular diseases. Research continues to find links between diabetes and development of disease not commonly assigned to hyperglycemia and its effects, including NASH cirrhosis, heart failure with preserved ejection fraction and renal disease without proteinuria. The diffuse prevalence of vascular destruction in potentially all organ systems and the interdependence of outcomes suggest that Diabetes should be viewed as a Cardiovascular-Renal-Metabolic disease. A consequence of this approach should be shifting from concentration on antihyperglycemic effects to evaluation of all therapies known to reduce poor outcomes in diabetics.
Research: Moving Beyond Standard of Care
Foundations: Standard of Care
Since its first publication, “Standards of Medical Care in Diabetes” 1988, the American Diabetes association has been the prominent reference in diabetes care. Updated annually, its evidence-based guidelines aid in medical decision making and (ideally) guide prescribing patterns of providers. These guidelines provide the “Standard of Care” protocol for treatment and have historically concentrated on achievement of intermediary measures such as HbA1c goals to treatment. Even within the past decade, guidelines have made tremendous strides in terms of not only appearance, but content as well. In Figure 1 below, we illustrate the “Standards of Medical Care in Diabetes” published in 2016.24
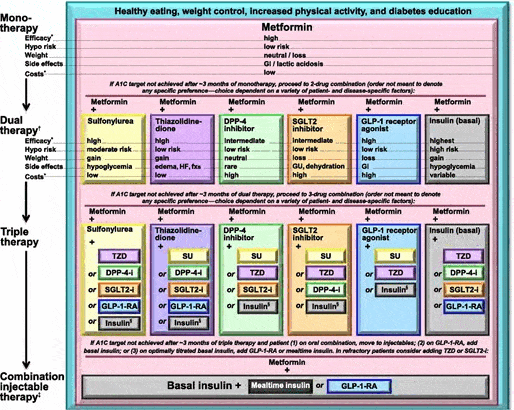
Figure 1. American Diabetes Association Standard of Care, 2016.
As illustrated in the figure, diabetes is viewed independently without consideration of the co-prevalence of disease or cost impact to the patient. Recommendations in the 2016 guidelines notably visually demonstrated a historical entrance to market of therapeutic classes from left to right rather than order based on efficacy of therapy.24 This cumbersome figure failed to provide a guideline-driven decision-making tool that continued to shift prescribing practice from eminence-based medicine to evidence-based.
In January, 2020 the ADA released their annual “Standards of Medical Care in Diabetes--2020” displayed below in Figure 2. Over the previous four years, the American Diabetes Association has adapted its guidelines to reflect the evidence from Cardiovascular Outcomes Trials previously focused on treatment of hyperglycemia. New patterns of treatment are being practiced in diabetic patients, in an effort to look beyond hyperglycemia and expand its inclusion of targeted disease states. This further illustrates diabetes as a complex cardio-metabolic renal disease.
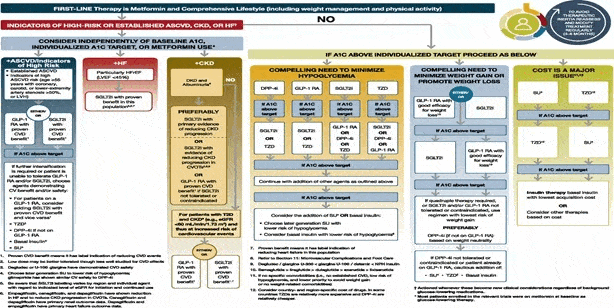
Figure 2. American Diabetes Association Standard of Medical Care in Diabetes, 2021.
Although the 2021 ADA guidelines include evidence of medication effects on specific disease states, they do not adequately emphasize advantages between classes. Addition of a “cost is a major issue” arm perpetuates the ability for sulfonylureas and thiazolidinediones to be viewed as potentially first line therapies further contributing to “therapeutic inertia”. Therapeutic inertia occurs when providers do not make timely adjustments to diabetic therapeutic regimens in uncontrolled diabetic patients.20,25 This could be a result of the inundation of diabetic research and guideline revisions over the previous decade, overchoice leading to inaction, often referred to as ‘decision paralysis’, or this could be a pattern of providers continuing to practice eminence based medicine. In our opinion published guidelines should direct treatment towards ideal pathways with as much specificity as possible. Practitioners should strive to obtain improved treatments for every patient. The realities of daily practice are problematic for every physician and lesser therapies should be sought only when best efforts are exhausted.
Sulfonylureas have significant potential for weight gain, hypoglycemia and cardiovascular disease. The risk is especially evident with advancing age. Current Beers criteria list long- acting sulfonylureas as medications to avoid in older adults regardless of diseases or conditions. The CAROLINA trial (2019) evaluated Linagliptin (DPP4) vs, Glimeperide (SU) for superiority of Linagliptin in cardiovascular outcomes. (primary outcome was not met) Study results were much anticipated to evaluate a sulfonylurea as an active competitor in a cardiovascular outcome trial. Results indicated that Glimepride did not increase cardiovascular outcomes. Further analysis reveals a potentially lower risk population with early onset of diabetes, no current insulin use and only 42% of the population had established cardiovascular disease. Hypoglycemic risk was significant with Glimeperide occurring 11.1X per 100 patient years vs 2.3X per 100 patient years with Linagliptin. Severe hypoglycemia was ~7x more likely and hypoglycemia leading to hospitalization was ~13X more likely to occur. The median age of participants was 64 years old indicating a population potentially at lower risk of hypoglycemic effects. While overall rates of hypoglycemia were low, the study population was at lower risk than typical clinic patients which would include insulin use as well as newer agents with greater antihyperglycemia effects. Even if interpreted to establish cardiovascular safety for Glimperide there still is the issue of inappropriate use of long acting sulfonylurea in elderly patients. It is not ideal to start therapies that need to be assessed for change starting at age 65 years old.
The addition of treatment arms indicated for a compelling need to minimize hypoglycemia and minimize weight gain or promote weight loss poses a medical oxymoron. As research continues to identify increasing cardiovascular risk associated with hypoglycemia and currently 85% of diabetics classified as overweight. We pose that nearly all diabetics should be included in these treatment arms. Further these are concerns best used in determining overall medication treatment recommendations and should be eliminated as a separate pathways.
The current guidelines advised three treatment paradigms for when ASCVD and HF or CKD predominate. The therapies acknowledge that consideration of medication implementation should be independent of baseline A1C, however it is not clear what treatment combinations should be sought when these conditions exist concomitantly. We propose that we move from the 2020 “when predominates” to “if present”. When a condition is present the targeted therapies may need to take precedence and be substituted for current medications. Effect in reducing poor outcomes exists in addition to glycemic reductions and therefore SGLT2 inhibitors and GLP1 agonsists should be evaluated for all opportunities to initiate. This could be with need for futher A1C reduction or without. Due to low rates of hypoglycemia there is less risk with newer combination therapies compared to previously more common regimens which included sulfonylureas and insulin.
By including acknowledgement of cardiovascular outcome trial data with regards to the SGLTi and GLP1 classes, the 2021 Standards do call to focus the gradual movement toward outcomes-based treatment – albeit still visually convoluted (or complicated) and potentially confusing. Diabetes is complex, the guidelines should be simple.
Solutions: Use Cardiovascular Outcome Trial (CVOT) Data to Guide Treatment
Cardiovascular Outcome Trials, commonly referred to as CVOT trials, in T2D are a result of a 2008 FDA Safety regulation that required all new anti-diabetic therapies to be studied in high-risk patients and focus on capturing safety issues. Specifically, the CVOT’s are designed to evaluate that the proposed therapy did not result in an increase in cardiovascular risk and demonstrate non-inferiority (that the therapy was not ‘unacceptably worse’).26 Each therapy developed after 2008 would be subject to conduct a CVOT. The outcome of conducting CVOT trials led to further research and development of trials to evaluate for superiority in therapeutic classes in specific disease states.
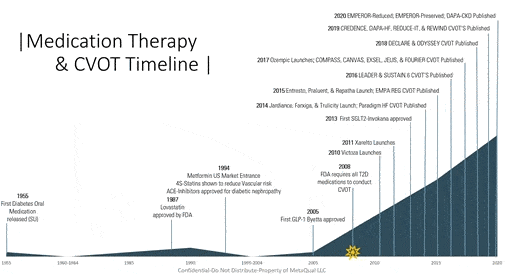
Figure 3- Medication Therapies and Cardiovascular Outcome Trial timeline 1955-2020.
To effectively evaluate both inferiority (harm) and superiority (compared to placebo), CVOT’s assess Major Adverse Cardiovascular Events, commonly referred to as MACE. MACE is based on a composite of events including Non-fatal MI, Non-fatal stroke, and CV death.26 The FDA does not regulate the risk study populations, so each CVOT demonstrates a different combination of risk patients ranging from at-risk to high-risk patients. The trials are concluded when the composite MACE endpoints are met leaving a significant number of questions about the true full benefits of these newer therapies. If studied for longer periods in at-risk patients, would therapies show a greater benefit of slowing the progression of disease or preventing a MACE altogether? This remains a topic of inquiry with precision medicine and may be further investigated in real-world studies in the future.
A recent publication produced from the American Diabetes Association co-sponsored by Eli Lilly and Novo Nordisk focuses on the cardiovascular outcome trials of antihyperglycemics.20 The authors focused attention to medication classes to treat diabetes in a patient-centric manner. This paper concentrates on medications with guideline-based recommendations for disease states co-prevalent in diabetics. In addition, consideration is given where compelling evidence exists but may not have a dedicated completed CVOT. This includes renal data associated with GLP1 medications. The studies included allow further analysis of treatments for parallel disease states that contribute to the Cardiovascular-Renal-Metabolic approach to Diabetes. By outlining multiple treatments across coexisting disease states there is hope that this will lead to increased collaboration of specialties.
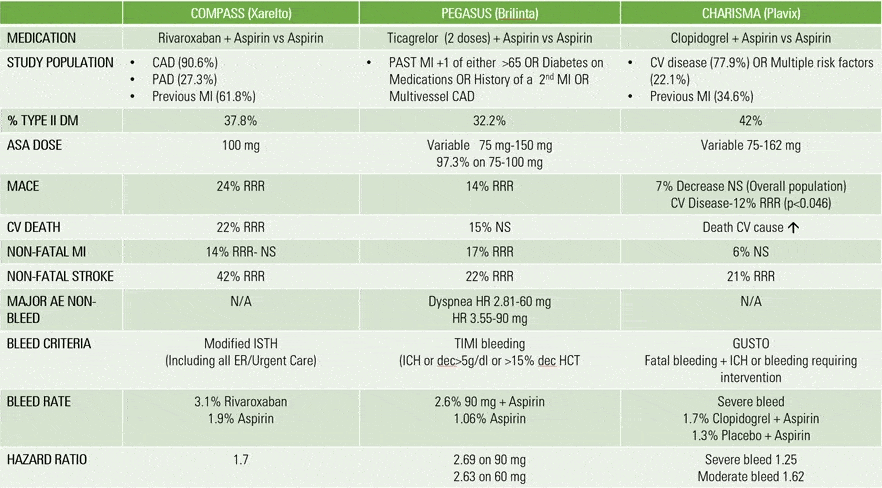
The intent of this paper is not to be an exhaustive review of every study available for the medications or class we include in our treatment paradigm. For example, HARMONY was not included due to Albiglutide is currently not available in the U.S. While metanalyses have shown potential for reduction in MACE for pioglitazone, we have chosen to not include this medication due to significant risk of weight gain, edema, bone loss and heart failure. Continued interest and practice patterns continue to exist for Dual Antiplatelet therapy (DAPT) in chronic stable cardiovascular risk. Also notably absent is CHARISMA or PEGASUS studies because in our analysis Rivaroxaban has a greater effect in reducing major cardiovascular outcomes (refer to Appendix B). Ezetimide was not included as there is a lack of trial evidence in the stable cardiovascular population. The greatest evidence for event reduction with Ezetimide was the IMPROVE-IT trial in which the population studied was in Acute Coronary Syndrome. Many of the outlined therapies have failed to show effect in the at-risk populations (primary prevention) compared to those with established cardiovascular disease (secondary prevention). Potential reasons include but not limited to non-standardization of definition of risk, multiple factors of varying risk and shorter length of trials with mixed populations. For example, it was long term follow up analysis of the UKPDS and DCCT trials which showed a decrease in macrovascular disease. The REWIND trial enrolled a low percentage of patients with established cv disease with mean follow up of 5.4 years. 27 Superiority was achieved for MACE outcomes in this population. The length of the trial may have contributed to this effect emerging in a mostly at risk population. Conversely, other included trials have shown impressive reductions in outcomes which led to early termination due to positive effect.
Residual Risk – Evidence From Cardiovascular Outcome Trials
In the EMPA-REG study which included >99% of patients with established cardiovascular disease (secondary prevention) there was a high percentage of standard of care included in the placebo arm– 95% on antihypertensive medication, 60% with BP controlled to <140/<90, 76% on lipid lowering therapy (statin or zetia) Despite this well represented population of standard care 12.% of this population experienced the primary outcome (CV death/nonfatal MI/nonfatal stroke) over a mean 3.1 yrs of observation. 28 In the REDUCE-IT trial in which 70.7% of the entry population had established cardiovascular disease, 29.3% had T2D and additional risk factors (high CV risk population) and 100% of patients were on background statin use (93% on moderate or high intensity), 22% of the study participants experienced the primary outcome (5 PT MACE) and 14.8% experienced the secondary endpoint (3 PT MACE) over 4.9 years of follow up. 29 In addition, the reduction in events with study drug (EPA) occurred in the subgroup of LDL<67 similarly to less controlled LDL suggesting risk persists even with LDL at aggressive targets.
T2D and CAD/MI/CV Death
Cardiovascular Outcome Trials of SGLT2 Inhibitors have lacked consistent effects in Myocardial Infarction or Death due to Cardiovascular cause. The CANVAS program (Canigliflozin) demonstrated reductions in all three MACE endpoints including nonfatal MI (15% RRR) which contributed to meeting superiority in the primary outcome (MACE -14% RRR).30 EMPA-REG (Empagliflozin) and DECLARE (Dapagliflozin) do show statistical downward trends of myocardial infarction but do not contribute greatly to the MACE risk reduction.31 The VERTIS CV trial did not reduce MACE, CV death or MI. EMPA-REG was notable for a 39% Relative Risk Reduction (RRR) in Cardiovascular death presumably related to a decrease in hospitalization for Heart Failure as myocardial infarction and nonfatal stroke were not reduced.28 The four completed Cardiovascular Outcome Trials of SGLT2 inhibitors have a wide disparity of cardiovascular risk in the study populations. The EMPA-REG study included >99% of patients with established cardiovascular disease.28 All of the study participants in the VERTIS CV trial had established atherosclerotic cardiovascular disease which could include coronary artery (76%)(prior MI – 48%), cerebrovascular or peripheral artery disease. The CANVAS trial included 65.6% and DECLARE included 40.6% of patients with established Cardiovascular disease. The definition of established cardiovascular disease and amount and type of risk factors used for identifying study candidates varied greatly between CANVAS and DECLARE.30,31 Further the DECLARE study excluded patients with eGFR <60.31 Direct comparison among trials with numerous differences in study population should be reserved. In applying an SGLT2 inhibitor for reduction in myocardial infarction consideration should be given to CANAGLIFLOZIN as myocardial infarction was a contributor in reaching superiority of the primary outcome in CANVAS.30,31 EMPAGLIFLOZIN and CANIGLIFLOZIN have strong evidence leading to FDA indication for reducing risk of Cardiovascular Death in patients with established cardiovascular disease. The only SGLT2 inhibitor currently FDA approved for reducing the risk of myocardial infarction in patients with established cardiovascular disease is CANAGLIFLOZIN.
Of the four GLP1 agonists available in the U.S two have shown reductions in myocardial infarction and two did not. LEADER (Liraglutide) and SUSTAIN-6 Semaglutide) demonstrated significant reductions in myocardial infarction which contributed to reaching statistical significance for the primary MACE outcome.32 SUSTAIN-6 had a 26% RRR of nonfatal MI. The LEADER study was notable in demonstrating a 22% RRR for Death from Cardiovascular cause and a statistically significant 15% RRR for Death from any cause. 33 The REWIND (Dulaglutide) and EXSCEL (Exenatide) studies did not demonstrate reductions in myocardial infarction.27 Similar to the SGLTi CVOTs there is a marked difference in the study design and populations. LEADER and SUSTAIN-6 both enrolled >80% of patients with established cardiovascular disease including Heart Failure.32 EXSCEL included 72% and REWIND only had 31.5% of the study population with a previous CV event. Definition of the at risk cohorts (Diabetes and risk factors) also varied greatly.27 Direct comparison of such disparate trials should be reserved. When evaluating use of a GLP1 agonist for reduction in Myocardial infarction or CV death LIRAGLUTIDE, SEMAGLUTIDE and DULAGLUTIDE have clear evidence in their Cardiovascular Outcome Trials.33
The REDUCE-IT trial was designed to test the potential superiority of treatment with Icosapent Ethyl vs placebo for MACE outcomes in patients at high CV risk with elevated triglyceride levels on background statin therapy.29 This was planned after analysis of the JELIS trial demonstrated superiority of Isocapent Ethyl and statin vs statin alone. (MACE – 19% RRR). Patients were required to have established cardiovascular disease or Diabetes with risk factors. 29.3% of patient had Diabetes at baseline with the vast majority Type 2. The Diabetes patients without cardiovascular disease were required to have at least one of 9 additional risk factors. Despite the wide range of degree of risk in the Diabetes patients REDUCE-IT demonstrated impressive reductions in cardiovascular outcomes.29 Statistical significance was achieved for superiority in the primary and secondary MACE outcomes. The trial showed relative risk reductions of: 26% for the primary outcome of death from cardiovascular cause, nonfatal MI, nonfatal stroke, coronary revascularization and unstable angina. 26% for the composite of death from cardiovascular cause, nonfatal MI and nonfatal stroke. 31% for the combined endpoint fatal and nonfatal MI. 20% for Cardiovascular Death. Additionally, there was a 31% RRR in Sudden Cardiac Death and 48% RRR in Cardiac Arrest. The effects were greatest in the secondary prevention group. The patients with and without Diabetes had similar outcomes. Reductions occurred irrespective of baseline triglyceride level or degree of reduction, suggesting mechanisms in addition to change in lipid fractions. All patients with Diabetes and Cardiovascular disease with elevated triglycerides (>150) should be evaluated for use of Isocapent Ethyl. Other trials of Omega-3-fatty Acids have not shown similar outcomes and caution should occur in assuming substitutions will confer the same cardioprotective effects. (VITAL 33)(STRENGTH 34)
PSK9i trials tested the hypothesis that LDL lowering in patients on statin therapy would reduce cardiovascular outcomes further. FOURIER (Alirocumab) and ODYSSEY OUTCOMES (Evolocumab) reached statistical significance for superiority of treatment in reducing MACE outcomes.35,36 Both trials had a primary outcome of cardiovascular death, nonfatal MI, nonfatal stroke, coronary revascularization and hospitalization for unstable angina. Both trials demonstrated significant reduction in myocardial infarction. (FOURIER 27% RRR – MI /ODYSSEY 27% RRR – Fatal/nonfatal MI) Additional outcomes of interest were a 22% RRR in Cardiovascular Revascularization in FOURIER and a 39% RRR in hospitalization for Unstable Angina in ODYSSEY OUTCOMES.35,36 The two studies have clinical treatment implications. First the reductions in MACE were seen with high rates of high intensity statin use in the background (70% of patients in FOURIER where on high intensity statin dosing and >80% of patients in ODYSSEY were on Atorvastatin 40 to 80mg or Rosuvastatin 20 to 40 mg).35 Second, reduction in MACE outcomes in FOURIER were significantly greater in year 2 of treatment compared to year one (CV death/nonfatal MI/nonfatal stroke – YR 1 – 16% RRR, YR 2 – 25% RRR). Third, despite both trials showing impressive reductions in LDL, ODYSSEY demonstrated the greatest effect in the patients with baseline LDL>100 and FOURIER demonstrated equivalent reductions irrespective of baseline LDL level.36 This difference may be explained by study design as the mean LDL level at study end in FOURIER was 30 mg/dL and in ODYSSEY there was a target LDL of 25-50 which allowed blinded medication adjustments resulting in a 48 month mean LDL of 66 mg/dl. It is clear that PSK9 inhibition reduces atherosclerotic cardiovascular disease outcomes in addition to statin therapy.36 This effect may increase over time and require LDL targets beyond reductions by statin intensity in current guidelines (cite AHA). All Diabetic patients with atherosclerotic cardiovascular disease should be assessed for LDL levels not at aggressive goals (<70) and evaluated for addition of PSK9 inhibition.
The COMPASS trial evaluated novel doses of the Direct Oral Anticoagulant Rivaroxaban alone and in combination with aspirin vs aspirin alone in patients with stable Coronary Artery Disease and Peripheral Artery Disease. 37 The COMPASS trial achieved the primary endpoint of decreasing MACE outcomes demonstrating a 24% RRR. The major contributor was 42% RRR in stroke. Other cardiovascular outcomes included a 14% reduction in myocardial infarction and 22% reduction in Death from Cardiovascular causes. While the reduction in myocardial infarction did not reach significance alone, the large reduction in ischemic stroke and additional decrease in Cardiovascular death demonstrate benefit to patients with stable Coronary Artery Disease. Rivaroxaban has been included in the 2021 ADA guidelines to be considered for patients with stable coronary and/or peripheral artery disease and low bleeding risk to prevent major adverse limb and cardiovascular events.
PARADIGM-HF (Sacubitril/Valsartan) was designed to evaluate Aldosterone – Neprilysin Inhibition vs Enalapril in the primary endpoint of Cardiovascular Death and hospitalization for Heart Failure.38 The trial included 60% of study patients with had a diagnosis of ischemic cardiomyopathy at time of randomization. The study was stopped early at interim analysis due to clear benefit of Angiotension – Neprilysin Inhibition. Cardiovascular death demonstrated a RRR of 20%. Hospitalization for Heart Failure demonstrated a 21% RRR. Clearly, diabetics with Heart Failure of ischemic origin could benefit from Angiotensin- Neprilysin Inhibition. Diabetics with HFrEF of ischemic origin should be evaluated for change from current RAAS inhibitor to Sacubitril/Valsartan.
T2D and STROKE
The CANVAS trials demonstrated effect across multiple vascular beds (coronary and cerebrovascular) which contributed to meeting the primary endpoint (3 POINT MACE).30 This includes a 10% reduction in nonfatal stroke and 13% reduction in the combination of fatal and nonfatal stroke. In contrast, reductions in stroke were not seen in other SGLT2i cardiovascular outcome trials. EMPA-REG demonstrated a statistical increase despite a 38% decrease in deaths assigned to cardiovascular cause. 28 Stroke reduction can only be assigned to Canagliflozin at this time.
Five cardiovascular outcome trials have been completed in the GLP1 class. Four of the studies met the pre-specified endpoint of noninferiority for cardiovascular risk.33,39The REWIND trial was prespecified to demonstrate superiority for MACE outcomes compared to placebo. Three trials demonstrated superiority to placebo in MACE outcome reduction. 27,33 In SUSTAIN-6 and REWIND the primary endpoint was met with the principal driver of reduced outcomes from stroke reduction. Endpoints in REWIND were 12% relative risk reduction for 3 POINT MACE and 27% for ischemic stroke.27 Endpoints in SUSTAIN-6 were 26% relative risk reduction for 3 POINT MACE and 39% for ischemic stroke. Semaglutide and Dulaglutide have significant evidence of reduction in stroke outcomes.33
The COMPASS trial evaluated Rivaroxaban and aspirin in combination with the prespecified goal to demonstrate superiority in MACE outcomes vs aspirin alone. Major adverse cardiovascular outcomes showed a 24% relative risk reduction.37 Stroke demonstrated a 42% RRR. This occurred despite a high baseline use of lipid lowering therapy (90%) and RAASi use (71.5%) It is clear that Rivaroxaban 2.5 mg and ASA 100 mg are superior to ASA 100 mg alone in reducing the risk of stroke. Major bleeding was increased overall but there was no significant difference in intracranial or fatal bleeding. Even with increased bleeding risk Death from any Cause was reduced 18%.
Prior to the PSK9 inhibitor Cardiovascular Outcome Trials evidence for stroke prevention outside of statin trials was limited and showed none or modest benefit. (ENHANCE and AIM HIGH). FOURIER and ODYSSEY demonstrated significant reduction in stroke risk in addition to baseline statin use. In FOURIER ischemic stroke was decreased by 21% in the treatment arm. In ODYSSEY the combined endpoint of fatal and nonfatal stroke was decreased by 22% in the treatment arm. Impressive reduction in LDL was achieved in both trials in addition to the baseline statin use (69.3% were on high intensity statin dosing in FOURIER).35,36 It is clear that further lipid lowering with PSK9i reduce stroke outcomes. The question exists whether the reductions seen are due to significant LDL lowering, final LDL level or other mechanisms.
REDUCE-IT demonstrated a 28% RRR in the combined endpoint fatal and nonfatal stroke.29 Diabetic patients with established cardiovascular disease or high risk of an ischemic stroke with Triglycerides >150 should be considered for treatment with Icosapent Ethyl.
T2D and CKD
In 2013 the first SGLT2 inhibitor Canagliflozin was approved for reducing hyperglycemia in Type 2 Diabetes. Little was known about the long term effects on renal function. Evidence from efficacy and safety studies suggested despite initial increase in creatinine and decrease in GFR there was a return to baseline. Cardiovascular outcome trials were designed with key secondary outcomes to assess renal effects. The CANVAS program included a second study (CANVAS-R) designed to assess the effects on albuminuria. Two key findings were found. CANVAS demonstrated a 27% reduction in the progression of albuminuria (defined as a >30% increase in albuminuria, change from normo to microalbuminuria or change from micro to macroalbuminuria).30 In addition there was a 40% reduction in the combined endpoint of a sustained 40% reduction in GFR, need for renal replacement therapy or death from renal cause. Further, there was 1.7X greater probability for regression of albuminuria. The DECLARE trial showed a 47% RRR with a composite renal outcome of sustained >50% reduction in eGFR to <60, End stage renal disease or death from renal cause. The majority of the effect was due to a >50% reduction in GFR to <60.31
Despite renal outcomes showing promise, there was still uncertainty as the study populations did not include a high percentage of CKD3 patients. For example in the CANVAS trial the mean eGFR was 76.5 and only 7.6% of the study population was identified as having macroalbuminuria. It was also not clear what the effects were of SGLT inhibiton on cardiovascular outcomes in patients with worsening renal function.30 In the CANVAS trial the primary outcome had a greater effect in the subgroup with eGFR 30 to <60. By contrast EMPA-REG showed the primary outcome and reduction in cardiovascular death to have a greater effect in the patients with eGFR 60 to <90.28,30
The CREDENCE trial was designed to evaluate the renal effects of Canagliflozin 100 mg vs placebo in patients with albuminuric chronic kidney disease who were on maximum tolerated RAAS inhibition.40 The primary composite outcome was defined as ESKD (dialysis/transplantation/sustained eGFR <15) or doubling of serum CRT or Death from renal or cardiovascular cause. Key secondary outcomes evaluated were MACE and hospitalization for heart failure. The study was stopped early at interim analysis due to clear benefit of Canagliflozin. The primary outcome (30% RRR) was met for superiority. The renal specific outcome of ESKD or doubling of serum CRT or Renal death was reduced by 34%. Multiple secondary outcomes showed significant reductions. 20% RRR in MACE, 39% reduction in hospitalization for heart failure, 22% reduction in CV death and a 17% reduction in Death from any cause. The study population was high risk for declining renal function and cardiovascular outcomes, exhibited by the mean eGFR of the study population was 56.2 and 50.4% had cardiovascular disease. The primary outcome had similar statistics across eGFR subgroups, potentially more effective with patients with <60 eGFR. CREDENCE was not an efficacy study with only a modest difference in reduction in HgbA1c by study end vs placebo.42 It is clear that glycemic control is unlikely to be the sole mechanism of SGLT2 inhibition to explain risk reduction in renal and cardiovascular outcomes. All patients with worsening renal function should be evaluated for SGLT2 inhibition. Canigliflozin is the first SGLT2 with an FDA indication to reduce the risk for end stage renal disease, worsening of kidney function, cardiovascular death and hospitalization for heart failure in patients with diabetic nephropathy and albuminuria >300 mg/day. DAPA-CKD has added evidence to benefit of SGLT2 inhibition in reducing poor renal outcomes. The study population included patients with GFR 25-75 and albuminuria with and without diabetes. This study was stopped early due to clear benefit of the treatment arm. The primary outcome of sustained decline in the estimated GFR of at least 50%, end-stage kidney disease or death from renal or cardiovascular disease was reduced by 44% (RRR) with a NNT of 19. Additionally death from any cause was reduced 31% (RRR). Greater cardiovascular event reduction may occur in patients with worsening renal function.
The LEADER and SUSTAIN-6 studies included prespecified secondary endpoints to evaluate effects on composite renal outcomes. LEADER had shown a 12% reduction in Nephropathy events (new onset macroalbuminuria/doubling of CRT and eGFR <45/need for RRT/Death from renal cause).33 The reduction was 30% RRR in those with eGFR <60. SUSTAIN-6 demonstrated a 36% RRR of the composite endpoint of New or Worsening Nephropathy (new persistent macroalbuminuria/doubling of CRT and eGFR <45/RRT). Liraglutide and Semaglutide have demonstrated reduction in renal outcomes. REWIND has further added evidence for new macroalbuminuria.
PARADIGM-HF designed to evaluate the superiority of Sacubitril/Valsartan vs Enalapril in Heart Failure and Cardiovascular Death included secondary safety endpoint of Time to first occurrence of a decline in renal failure (ESRD/decrease in eGFR of at least 50%/decrease of >30 ml /min for a reduction to <60 ml/min).39 94 patients in the Sacubtril/Valsartan met the endpoint and 108 in the Enalapril arm (nonsignificant). The reduction in primary endpoints was similar in the subgroup of baseline CRT<60. PARADIGM-HF establishes renal safety of Angiotensin- Neprylisin Inhibition.38
REDUCE-IT did not include prespecified renal outcomes. The primary MACE outcomes (cardiovascular death/nonfatal mi/nonfatal stroke/coronary revascularization/unstable angina) were reduced in all creatinine subgroups.29 The lowest tertile (GFR<60) demonstrated the greatest reduction with a 29% reduction of the primary outcome. This is an important finding demonstrating efficacy in a very high risk group of patients with HFrEF and poor renal function.
T2D and HF
SGLT2 inhibitors have shown consistent effect with reductions in hospitalization for heart failure. Both EMPA-REG (35% reduction hHF) and the CANVAS trials (33% reduction in hHF) showed significant reductions. This led to the redesign of DECLARE while the trial was ongoing to include a new combined primary endpoint of Cardiovascular Death and hospitalization for Heart Failure.28,30,31 Declare did not meet criteria for superiority vs. placebo in MACE but did meet significance for its second primary endpoint.31
Study design and trial populations differ greatly between these 3 cardiovascular outcome trials. This lends uncertainty as to what effect fatal and nonfatal heart failure hospitalizations contribute to improvements in cardiovascular death. EMPA-REG decrease in CV death outcomes was thought to be driven by reduction in Heart Failure. 28 This pattern did not occur in CANVAS or DECLARE. In CANVAS myocardial infarction, stroke and heart failure reductions contributed to the 13% decrease in CV death. In DECLARE despite a 27% reduction in hHF there was no significant difference in CV outcomes between dapagliflozin and placebo. 30,31 It is important to note it is not consistent across studies whether patients with a history of heart failure prior to entering the study fair better with regards to cardiovascular outcomes. Another important finding was a reduction in hHF in patients without a prior history. The CANVAS trial demonstrated a 24% reduction in this subgroup.30 This finding suggest the importance of identifying subclinical disease/emerging risk and targeting patients at high risk of heart failure with the appropriate therapies.
The CREDENCE trial designed to evaluate renal outcomes in a very high risk population further evaluated heart failure outcomes.40 Again a clear benefit was seen (39% reduction hHF) despite a significant reduction in eGFR at baseline (mean eGFR 56.2). Cardiovascular death and Death from any cause were reduced 22% and 17% although both of these did not reach statistical significance.
DAPA-HF was designed to evaluate the effects of SGLT2i with Dapagliflozin in patients with and without T2D. Patients with low ejection fraction heart failure (EF <40%) were randomized to dapagliflozin or placebo. 41 The primary endpoint of worsening heart failure, defined as hospitalization or urgent visit requiring I.V. therapy, met significance for superiority. The reduction in the primary endpoint, cardiovascular death and death from any cause were statistically significant and similar irrespective of the presence of T2D. More recently, the EMPORER-REDUCED and EMPORER-PRESERVED were designed to evaluate for primary outcome for heart failure hospitalizations and cardiovascular death in a diverse ejection fraction patient population with and without diabetes as a comorbidity.42,43 Both trials showed significant reduction in the primary endpoint driven mostly by reductions in hospitalization for heart failure. These studies further advance evidence that the marked effect of SGLT2i with reductions in heart failure is not primarily from glycemic reductions.
In all of these trials the greatest statistical effects on specific disease process were in the Renal and Heart Failure outcomes. There are significant links between these comorbidities and complications especially with risk of increasing morbidity and mortality in T2D. The prevalence of these comorbidities individually or in combination should require identifying proper candidates for SGLT2 inhibition.
GLP1 agonist CVOTs have failed to show improvements in heart failure. The LEADER trial evaluated hospitalization for heart failure as a secondary endpoint showing a non statistically significant reduction of 13%.33 Two subsequent studies have included hospitalization for heart failure (hHF) endpoints. SUSTAIN-6 included 23.6% of the study population with a history of Heart Failure. Despite the inclusion of this high-risk group there was no reduction in hHF. EXSCEL did not use Heart Failure as a study entry condition but did evaluate hHF. Again, there was no reduction in this endpoint. The primary endpoint which met criteria for noninferiority but not superiority did not show a difference in the patients with or without Heart Failure.39
PARADIGM-HF was designed to show superiority of Sacubritil/Valsartan vs. Enalapril in the combined outcome measure of Death from Cardiovascular cause and hospitalization for Heart Failure in patients with low ejection fraction heart failure. 34.6% of the trial population had Diabetes.44 The primary endpoint met criteria for superiority. Cardiovascular death decreased 20% and hHf decreased 21%. In addition, death from any cause showed a 16% RRR. There were similar outcomes with the primary endpoint in patients with and without Diabetes. Paradigm-HF was stopped early at interim analysis due to clear benefit of Angiotensin-Neprilysin inhibition.45 Recently, PARAGON-HF evaluated the primary composite endpoint of hospitalizations for heart failure and cardiovascular death in patients with preserved ejection fraction. There was a 13% reduction in primary endpoint driven by reduction in hospitalization for heart failure.46 The patients who appeared to benefit the most were those in the mid-range ejection fraction cohort. In patients with T2D and Heart Failure with low to mid-range ejection fraction there should be consideration of replacing the current RAAS inhibitor with the combined medication of Sacubritil/Valsartan.
The ODYSSEY trial included 14.9% of patients with a history of heart failure.36 There was no statistical difference in secondary outcome of hospitalization for heart failure in the total study population. PSK9 inhibition could be considered if there is an atherosclerotic etiology of low ejection fraction but not as a primary treatment for heart failure.
DM2 and PAD
The patient with Type 2 Diabetes and PAD are at high risk of hospitalization, death from cardiovascular cause and major adverse limb events (amputation and interventions) (JACC – Anand et al – COMPASS subanalysis). The COMPASS trial was designed to evaluate the effects of Rivaroxaban alone and Rivaroxaban and aspirin compared to aspirin alone. The COMPASS trial enrolled 27,395 patients of which 37.7% had T2D and 27.3% had PAD.37 There was a 24% RRR of the primary endpoint (CV death/MI/Stroke) statistically significant for superiority in the Rivaroxaban and aspirin treatment arm. Patients with PAD at baseline showed a greater reduction (28%) in the primary outcome. There was a 43% RRR in the combined endpoint of Severe Limb Ischemia and Major Vascular Amputation (MALE). Additional outcomes include a 58% reduction in total vascular amputations and 24% reduction in peripheral vascular intervention. It is clear that Rivaroxaban and aspirin are superior to aspirin alone in preventing MACE and MALE outcomes in the PAD subgroup.
In the CANVAS trials the subgroup of patients with peripheral vascular disease at baseline had greater reductions in the primary endpoint (MACE). Safety analysis in the CANVAS program demonstrated an approximate doubling in the risk of amputations. This complication had not previously been seen in previous efficacy and safety studies of SGLT2 inhibitors including Canagliflozin. The CREDENCE trial was ongoing when CANVAS was published and subsequently was modified for foot examination at each encounter and temporary cessation of treatment if any condition was identified that could lead to amputation.30,40 This was in response to data showing a large majority of the patients with amputations occurring during the CANVAS trial had a history of amputation, active lower extremity Diabetic foot infections or known PAD.30 Amputation type was similar in the Canagliflozin and placebo populations. Subsequent database metanalysis have been mixed. (cite OBSERVE 4-D/Chang et al/Ueda et al) A second medication in this class Ertugliflozin has shown statistical increase in amputations. Proposed mechanism has been related to volume depletion, especially in the periphery. Recent evaluation of increased risk for amputation with diuretic use in diabetics has been demonstrated. (SURDIAGNE Study) It is probable that the patient with high risk of amputation (severe PAD and active lower extremity DFIs) should not have SGLT2 inhibitors initiated. In addition it is prudent to confirm patients are well hydrated and evaluated for reduction in dosing of diuretics at the time of initiating an SGLT2 inhibitor.
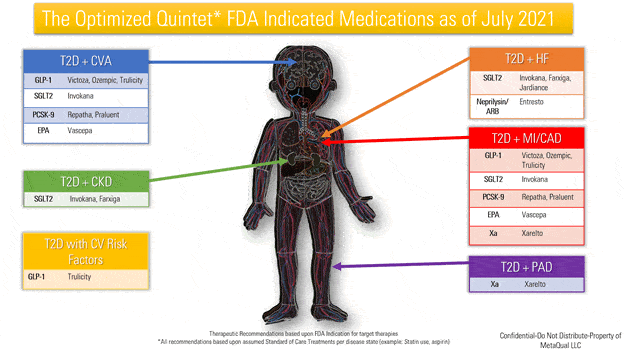
The “Optimized Quintet”: A Pathway to Treatment*
The concept for the Optimized Quintet is derived from DeFronzo’s Ominous Octet in which the effects of 8 organ systems contribute to hyperglycemia in diabetes.2 In our pathway, we attempt to illustrate how therapies impact the outcomes of diabetes. The goal of the Optimized Quintet is to advance therapy beyond current standards, increase proper guideline-based use of emerging therapies and improve outcomes in the high-risk patients with significant residual risk. This illustration includes class of medication (except Rivaroxaban and Sacubitril/Valsartan) to identify therapies for treatment optimization.
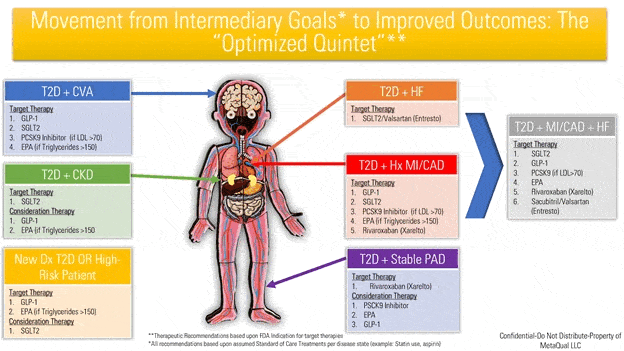
*M Attanasio DO and M Santanna, MA 2020.
(Appendix A – Identifies the medications within class with FDA approval for use in each comorbidity and complication.) What is not presented visually is current well-established standards of care. For example, RAAS inhibition in Diabetic nephropathy, statin use in cardiovascular disease, hypertension control, blood glucose control, or the importance of proper diet. In addition, what is proposed is not meant to replace established guidelines in current acute disease states, including but not limited to acute coronary syndrome, a recent stented coronary artery or acute cerebrovascular accident. We do propose that when treatment plans are made in acute disease states that subsequent plans are made for change to improved chronic therapies. As an example a patient who has had multiple admissions for heart failure should have a plan upon discharge for initiation of a SGLT2 inhibitor. Another example would be a patient with coronary artery disease who has a stent placed in the lower extremity. DAPT therapy may be indicated initially but there should be a plan to initiate chronic low dose Rivaroxaban at a specified time. Further hope exists that including these therapies as a treatment paradigm will increase collaboration among key specialties and primary care.
All recommended therapies should be implemented for each comorbidity and complication if proper criteria exist. Patients who have multiple comorbidities or complications are at high risk of recurrent readmission, worsening morbidity and mortality. By considering CVOT outcome data and guideline-based recommendations we are proposing a comprehensive care pathway that aims to reduce therapeutic inertia by illustrating a clear pathway to advanced therapies.
Target Therapy
A target therapy is a medication or class that has proven significant improvement in outcomes with a corresponding FDA indication. An example would be SGLT2i with Invokana 100 mg – based on the CREDENCE trial – in patients with Diabetic nephropathy and albuminuria.42 Reductions in renal and cardiovascular outcomes occurred with only modest reduction in HgbA1c suggesting mechanisms in addition to glycemic improvement.
Consideration Therapy
A consideration therapy is a medication or class that has strong evidence but does not have an FDA indication. An example would be GLP1 agonists with evidence reducing renal endpoints. Liraglutide/LEADER and Semaglutide/SUSTAIN-6 included evidence in reduction in renal outcomes that reached significance as secondary endpoints.
Population Health Recommendations
Over 34 million Americans live with diabetes today and this population is expected to be nearly 42 million by 2030.47,48 As the incidence and prevalence continues to rise and co-morbidities develop, the burden of care for these patients will strain the healthcare system in the US.49 This increasing requirements for care for diabetics will reduce healthcare access leading to treatment delays and delays in screening and prevention and ultimately increase chronic care management efforts. Understanding the CV disease risks early and often for diabetics while simultaneously working toward primary and secondary prevention strategies can help to ease the burden on the health care system. We aim to focus practice on three important goals:
• Deliver Incremental Care
• Implement optimized treatment pathways
• Develop a Multi-inter-disciplinary Implementation Program (MIP)
Deliver Incremental care
One of the most influential paradigm shifts in healthcare over the previous decade has been the deliberate movement from episodic, non-preventative care to incremental, value-based care. This transformation was largely shaped through the Nation Committee on Quality Assurance’s (NCQA) Patient Centered Medical Home model which reintroduced the concept of preventative and incremental care through an omni-channel achievement approach. The PCMH model carefully crafted important delivery, access and coordinated care requirements that shaped practice operations today.
In adopting the PCMH model as a foundation of practice, this allows for the movement from fee-for-quantity payments to fee-for-value payments providing sustainability for primary care practices and increases delivery of high quality, patient-centric care. The focus on meaningful encounters promotes increased patient education, engagement, and ideally medication adherence. Partnering with patients using a realistic and evidence-based care plan with medications that not only lower risks of multiple adverse events but also offer health benefits like weight loss, decreased blood pressure, and even possible eventual deprescribing.
Incremental visits cultivate an open dialogue and help to identify patient non-adherence and encourage opportunities to work together through the challenges of prior authorizations and the ever-changing formulary updates to further optimize care plans. This allows for more frequent touchpoints to stress the importance of self-management, reiterate diet and lifestyle choices and offer opportunities for improved care management.
Implement Optimized Treatment Pathways
The cost of care for the complexity of diabetes does not come with a small price tag. One in every four US healthcare dollars are spent on diabetic care.50 In 2017, $327 billion dollars were spent on diabetic care in the US alone.51 The cost of care and spending are exponentially increasing. The dollar amount spent on diabetes from 2012-2017 increased by 26%.50 Poorly controlled diabetes is also associated with higher total cost of care compared to well controlled patients, including costs of hospitalization and medication costs.52 In 2015 more than 12 million ER visits were by patients with diabetes over the age of 45, accounting for 25% of all ER visits for the entire year.53 This rate is 6 times higher than non-diabetic patients.53 This rate continues to increase with age, with the highest ER utilization for diabetics found in patients 75 years and older.53 30% of diabetics will visit the ER in a given year.54 Diabetic patients are more likely to be admitted to the hospital (78% of the time in persons over 45 with diabetes; 24% more likely compared to non-diabetics). 24.8% of all US hospitalized days in 2017 were incurred by diabetic patients, and over half (13.9%) were attributed directly to diabetes.51
These trends in diabetic utilization are unsustainable. Unsustainable for the health care system. Unsustainable for patient lives. There have been an eruption of new therapies supported by large randomized controlled trials with reductions in cardiovascular outcomes. The first step to reducing utilization is to begin using the clinical evidence to drive an optimized treatment pathway. The purpose of CVOT is to differentiate treatments that improve health outcomes. Implementation of optimized treatment framework benefits both patients and providers by driving down ER and hospital utilization costs, improve intermediary measures like HbA1c, and improve health outcomes for patients.
It would be remiss to say implementing these new therapies will not be challenging from multiple aspects. One factor that is largely dismissed is the under-utilization of evidence-based guidance in practice as discussed earlier in the paper. If disparity of knowledge is prevalent, and information is unavailable through open-access pharmaceutical relationships, providers are left to actively seek information independently. This can also lead to therapeutic inertia and stagnant prescribing habits resulting in increased costs of care to healthcare and poor health outcomes for patients.
Additional challenges to treatment optimization include therapeutic access, coverage, and most importantly, affordability through payers and PBMs (Pharmaceutical Benefit Managers). One way to accomplish these lofty goals is through creative value-based partnerships with pharmaceutical companies that are driven by the total cost of care. These value-based partnerships are structured so the goals of treatment are established on evidence based guidelines to demonstrate improved health outcomes and reduction in total cost of care. UPMC Health Plan and Boehringer Ingelheim (BI), the producers of Jardiance, demonstrated success in improved health outcomes and as a result reduced the total cost of care in their diabetes-driven value-based partnership. BI confidently entered into an at-risk contract with UPMC Health Plan that stated if Jardiance did not result in total cost of care savings compared to other T2D treatment in the appropriate patient population by the end of their contract, BI would retrospectively adjust the net price of Jardiance. The study sample included 134,599 non-Jardiance participants and 2112 Jardiance participants. At the end of UPMCHP and BI’s contract the total cost of care for Jardiance vs non-Jardiance patients was a savings of $13,704 ($42,384-non-Jardiance patients vs $28680-Jardiance patients) per patient per year. The biggest driver of savings was medical costs, with a 50% reduction realized. The innovative partnership builds the foundation for this model to be replicated.
Expanding on Pharmaceutical-Payer innovative agreements, if cost savings are realized utilizing optimized therapies alignment with goals of value-based payer-provider contracts must follow. This reimagination of quality incentives based upon outcomes not intermediary measurements will encourage practitioners to utilize optimized therapies to achieve. The top-down value-based relationship model propels the goals of the quadruple aim.
Develop a Multi-inter-disciplinary Implementation Program (MIP)
Initiating comprehensive care coordination may aid in reducing the clinical inertia often experienced between specialists and primary care practitioners who defer to each other for treatment planning. Collectively standardizing treatment protocols strengthens the ability to carry forward evidence-based care plans and allows patients and providers positively impacting health outcomes. Shifting from traditional fee-for-service to a fee-for-value based model for both PCP’s and specialists fosters a collaborative relationship and can be immensely impactful in both cost and health outcomes.
Applying an innovative model for collaboration in the cardiovascular metabolic renal diabetic patient population is an important point of origin for an initiative. It can be imagined in 3 essential phases. Implementation science strategies would lend to the development of a multi-interdisciplinary team (MIT) to develop a therapeutic coordination initiative. Inclusive in this MIT, is built from the key stakeholders of the pharmaceutical industry, an engaging payer, a PBM, a population health management company, primary care providers, cardiologists, nephrologists, endocrinologists, vascular physicians, pharmacists, care managers and IT support. In phase one, the selection of specialists and MIT members would be rooted in their ongoing efforts in population health and have demonstrated performance in quality and outcomes-based programs. This team’s first task would be to collectively evaluate new evidence from CVOT’s and the optimized quintet pathway illustrated earlier. Using this model as a guideline-based, streamlined protocol for application in the stable cardiovascular metabolic renal diabetic patient this can begin to gain a collective consensus for a multi-disciplinary treatment pathway. Emphasis will be adding treatment paradigms for newer therapies in addition to current standard of care.
Also, during phase one, expectations from the pharmaceutical companies, PBM, and payer include not only mutually agreed upon high-value outcome-based (probable risk-based) contracts like the UPMCHP and BI model. Additionally, ensuring both accessibility and affordability to patients is a key component of this program. This is a critical step in implementing a program with health outcomes in mind. If cost is minimized as a barrier to the patient, this opens the window of opportunity to provide previously unavailable treatments, engage patients in a new meaningful way, potentially increase medication adherence, improve health outcomes while decreasing the cost of care. Finally, with the quadruple aim in mind—incentivization to providers will ultimately be a large driving force in this initiative. Providers strive to provide the best care for their patients but all too often are caught in the administrative apathetic churn of prior authorizations and seemingly unnavigatable realm of medication coverage. A consensus-driven simplistic pathway that is intentionally specified in the value-based agreements will have the greatest likelihood for milestones of success in the quadruple aim.
Once consent on the pathway is accomplished, this begins phase two and will create an omnichannel experience for providers and patients with a unified and repeated process to create efficiency and safety with the ultimate goals of improved health outcomes and reduced cost of care. To realize these goals, it is important to critically evaluate the next steps of implementation and ensure open, frequent means of communication between all members of the teams and their respective organizations. Identifying key accountable contacts in each specialty, PCP office and organization for operational issues is paramount to both viability and sustainability of the initiative.
Robust management and oversite using implementation science, program management and IT must be designed and deliberate milestones and reporting cadence to monitor progress would be defined at the onset of the initiative. Leadership engagement from the team regularly regarding reports would help to steer the initiative, guide observed process issues, and monitor progress of the overall project. This pioneer multi-disciplinary team would serve an initial term of three years to effectively allow for the implementation, education, monitoring, process improvement, and maintenance phase. Phase three would consist of the evaluation and re-imagination phase. In this phase, the development of best practices, lessons learned and critical re-evaluation of the initiative to consider duplication in other geographic markets across the US. Expansion of the treatment pathway to include additional co-morbidities such as obesity, metabolic syndrome, and NASH cirrhosis as well as improved early identification of emerging cardiovascular metabolic renal risk. This important research could help to shape the future of precision medicine.
Lasting Thoughts
This paper aimed to demonstrate that the medical community should no longer think of diabetes in a paradigm of treatment for hyperglycemia, but rather consider co-morbid conditions as targets for therapy to reduce the cardiovascular outcomes of the patient. Progressing forward past standard of care and understanding the incredible amount of CVOT studies that have been published in the previous decade lead a course to a newer inclusive, simplistic, and optimized treatment pathway that guides providers to treating cardiovascular metabolic renal diabetic patients collectively.
The “Optimized Quintet” intends to unpack the difficulty of the complex co-morbid patient into a streamlined diagram. By implementing this evidence-based treatment pathway, we are aiming to invest in the care of our patients today and working to improve health outcomes and reduce cost of care to actually achieve in the quadruple aim for tomorrow. The downstream impacts of using this therapeutic pathway would encourage providers to move beyond the therapeutic inertia, and to challenge and encourage both evidence-guided value-based contracting in support of a person-specific movement toward precision medicine.
Finally, the implementation science of population health integration is essential to taking this pathway from paper to practice. Development of a multi-inter-disciplinary team (MIT) to guide a phased value-based initiative will be necessary to drive the research for demonstrating the efficacy of this pathway to truly understand the total cost of care and health outcomes in the cardiovascular metabolic renal diabetic patient population.
Appendix
Appendix-A (FDA Indications Chart & FDA Indications by Therapeutic Class)
FDA Indications by Therapeutic Class
SGLT2
INVOKANA/CANIGLIFLOZIN
· To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease
· To reduce the risk of end-stage kidney disease, doubling of serum creatinine, cardiovascular death, and hospitalization for heart failure in adults with type 2 diabetes mellitus and diabetic nephropathy with albuminuria
JARDIANCE/EMPAGLIFLOZIN
· To reduce the risk of cardiovascular death in adult patients with type 2 diabetes mellitus and established cardiovascular disease
· To reduce the risk of cardiovascular death plus hospitalization for heart failure in adults with heart failure with reduced ejection fraction (HFrEF)
FARXIGA/DAPAGLIFLOZIN
· To reduce the risk of hospitalization for heart failure in adults with type 2 diabetes mellitus and established cardiovascular disease or multiple cardiovascular risk factors
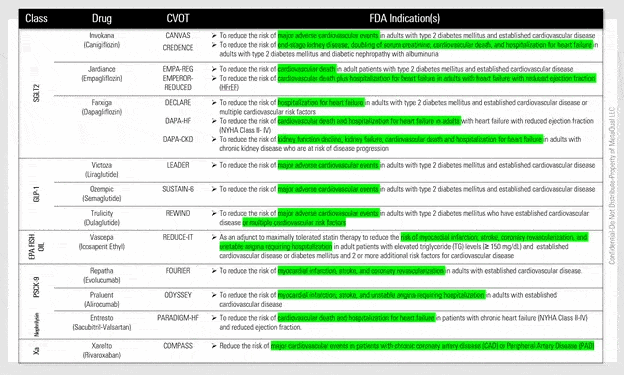
· To reduce the risk of cardiovascular death and hospitalization for heart failure in adults with heart failure with reduced ejection fraction (nyha class ii- iv)
· To reduce the risk of kidney function decline, kidney failure, cardiovascular death and hospitalization for heart failure in adults with chronic kidney disease who are at risk of disease progression
GLP-1
VICTOZA/LIRAGLUTIDE
· To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease
OZEMPIC/SEMAGLUTIDE
· To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus and established cardiovascular disease
TRULICITY/DULAGLUTIDE
· To reduce the risk of major adverse cardiovascular events in adults with type 2 diabetes mellitus who have established cardiovascular disease or multiple cardiovascular risk factors
EPA Fish Oil
VASCEPA/ICOSAPENT ETHYL
· As an adjunct to maximally tolerated statin therapy to reduce the risk of myocardial infarction, stroke, coronary revascularization, and unstable angina requiring hospitalization in adult patients with elevated triglyceride (TG) levels (≥ 150 mg/dL) and established cardiovascular disease or diabetes mellitus and 2 or more additional risk factors for cardiovascular disease
PSCK9 Inhibitors
REPATHA/EVOLOCUMAB
· To reduce the risk of myocardial infarction, stroke, and coronary revascularization in adults with established cardiovascular disease.
PRALUENT/ALIROCUMAB
· To reduce the risk of myocardial infarction, stroke, and unstable angina requiring hospitalization in adults with established cardiovascular disease
Neprilysin Inhibitors
ENTRESTO/SACUBITRIL-VALSARTAN
· To reduce the risk of cardiovascular death and hospitalization for heart failure in patients with chronic heart failure (NYHA Class II-IV) and reduced ejection fraction.
Factor Xa inhibitors
XARELTO/ RIVAROXABAN
· Reduce the risk of major cardiovascular events in patients with chronic coronary artery disease (CAD) or Peripheral Artery Disease (PAD)
Appendix B
References
- World Health Organization. (Chan M, ed.). Geneva, Switzerland: World Health Organization; 2016:1–88. https://apps.who.int/iris/bitstream/handle/10665/204871/9789241565257_eng.pdf;jsessionid=518A865F3D72E414CA92898835BCDE48?sequence=1
- DeFronzo RA. (2009) From the Triumvirate to the Ominous Octet: A New Paradigm for the Treatment of Type 2 Diabetes Mellitus. Diabetes. 58(4): 773–795. doi:10.2337/db09-9028
- Lau LH, Lew J, Borschmann K, Thijs V, Ekinci EI. (2018) Prevalence of diabetes and its effects on Stroke outcomes: A meta-analysis and literature review. J Diabetes Investig. 10(3): 780–792. doi:10.1111/jdi.12932
- American Diabetes Association. (2019). Standards of Medical Care in Diabetes—2020 Abridged for Primary Care Providers. Clin Diabetes. 38(1): 10–38. doi:10.2337/cd20-as01
- Chen R, Ovbiagele B, Feng W. (2016) Diabetes and Stroke: Epidemiology, Pathophysiology, Pharmaceuticals and Outcomes. Am J Med Sci. 351(4):380–386. doi:10.1016/j.amjms.2016.01.011
- Echouffo-Tcheugui JB, Xu H, Matsouaka RA, et al. (2016) Diabetes and long-term outcomes of ischaemic stroke: findings from Get With The Guidelines-Stroke. Eur Heart J. 2018;39(25):2376-2386. doi:10.1093/eurheartj/ehy036
- Heart Association A. (2019) Diabetes and heart failure are linked; treatment should be too. www.heart.org. https://www.heart.org/en/news/2019/06/06/diabetes-and-heart-failure-are-linked-treatment-should-be-too. Published June 6, 2019. Accessed June 7, 2020.
- Mcallister DA, Read SH, Kerssens J, et al. (2018) Incidence of Hospitalization for Heart Failure and Case-Fatality Among 3.25 Million People With and Without Diabetes Mellitus. Circ. 138(24): 2774–2786. doi:10.1161/circulationaha.118.034986
- Cavender MA, Steg PG, Smith SC, et al. (2015) Impact of Diabetes Mellitus on Hospitalization for Heart Failure, Cardiovascular Events, and Death. Circ. 132(10): 923–931. doi:10.1161/circulationaha.114.014796
- National Diabetes Education Program. Snapshot of Diabetes. 2011. https://www.nkfm.org/sites/default/files/documents/ndep_diabetes_snapshot.pdf. Accessed June 25, 2020.
- Whiting DR, Guariguata L, Weil C, Shaw J. (2011) IDF Diabetes Atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 94(3): 311–321. doi:10.1016/j.diabres.2011.10.029
- Einarson TR, Acs A, Ludwig C, Panton UH. (2018) Prevalence of cardiovascular disease in type 2 diabetes: a systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc Diabetol. 17(1). https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5994068/#:~:text=%5B6%5D%20reported%20death%20rates%20due,having%20a%20history%20of%20MI. doi:10.1186/s12933-018-0728-6 Accessed June 8, 2020.
- Thiruvoipati T. (2015) Peripheral artery disease in patients with diabetes: Epidemiology, mechanisms, and outcomes. World J Diabetes. 6(7): 961. doi:10.4239/wjd.v6.i7.961
- Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and Its Burden in the United States, 2014, Atlanta, GA: US Department of Health and Human Services; 2014. http://www.cdc.gov/diabetes/data/statistics/2014statisticsreport.html
- Wu S, Driver V, Wrobel J, and Armstrong D. (2007) Foot Ulcers in the Diabetic Patient, Prevention and Treatment. Vasc Health Risk Manag. 3(1): 65–76.
- Pemayun TGD, Naibaho RM, Novitasari D, Amin N, Minuljo TT. (2015) Risk factors for lower extremity amputation in patients with diabetic foot ulcers: a hospital-based case–control study. Diabet Foot Ankle, 2015. 6:10.3402/dfa.v6.29629. doi:10.3402/dfa.v6.29629. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4673055/ (accessed 8/5/2016)
- Yazdanpanah L, Nasiri M, Adarvishi S. (2015) Literature review on the management of diabetic foot ulcer. World J Diabetes. 6(1): 37–53. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4317316/
- Humphries MD, Brunson A, Hedayati N, Romano P, Melnkow J. (2016) Amputation Risk in Patients with Diabetes Mellitus and Peripheral Artery Disease Using Statewide Data. Ann Vasc Surg. 30: 123–131. doi:10.1016/j.avsg.2015.04.089
- National Institute of Diabetes and Digestive and Kidney Diseases N. Kidney Disease Statistics for the United States. National Institute of Diabetes and Digestive and Kidney Diseases. https://www.niddk.nih.gov/health-information/health-statistics/kidney-disease. Published December 1, 2016. Accessed July 8, 2020.
- Harris SB, Cheng AYY, Davies MJ, et al. (2020) Person-Centered, Outcomes-Driven Treatment: A New Paradigm for Type 2 Diabetes in Primary Care. Arlington (VA): ADA. PMID: 32667763. https://www.ncbi.nlm.nih.gov/books/NBK559432/
- Burrows NR, Hora I, Geiss LS, Gregg EW, Albright A. (2017) Incidence of End-Stage Renal Disease Attributed to Diabetes Among Persons with Diagnosed Diabetes — United States and Puerto Rico, 2000–2014. MMWR Morb Mortal Wkly Rep. 66(43):1165-1170. doi:10.15585/mmwr.mm6643a2
- University of California San Francisco. Statistics · The Kidney Project. The Kidney Project. https://pharm.ucsf.edu/kidney/need/statistics. Published February 20, 2020. Accessed July 8, 2020.
- CMS. Centers for Medicare & Medicaid Services. NHE Fact Sheet. CMS. https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet. Published March 3, 2020. Accessed July 8, 2020.
- American Diabetes Association. (2015) Standards of Medical Care in Diabetes—2016: Summary of Revisions. Diabetes Care. 39(Supplement 1). doi:10.2337/dc16-s003
- Khunti S, Khunti K, Seidu S. (2019) Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab. 2019:10:2042018819844694
- American Association of Clinical Endocrinologists, A. (2018, November 18). Cardiovascular Outcomes Trials in Types 2 Diabetes. In Cardiovascular Outcomes Trials in Type 2 Diabetes. Retrieved June 8, 2020, from https://www.aace.com/sites/default/files/201902/CVOT_Outcome_Trials_in_T2DM_111818_reduce.pdf
- Gerstein HC, Colhoun HM, Dagenais GR, et al. (2019) Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double‐blind, randomised placebo‐controlled trial. Lancet. 394(10193):121–130.
- Zinman B, Wanner C, Lachin JM, et al. (2015) Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med. 373(22): 2117–2128. doi:10.1056/nejmoa1504720
- Bhatt DL, Steg PG, Miller M, et al. (2019) Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N Engl J Med. 380(1): 11–22. doi:10.1056/nejmoa1812792
- Neal B, Perkovic V, Mahaffey KW, et al. (2017) Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N Engl J Med. 377(7): 644–657. doi:10.1056/nejmoa1611925
- Wiviott SD, Raz I, Bonaca MP, et al. (2018) Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 380(4): 347–357. doi:10.1056/nejmoa1812389
- Marso SP, Bain SC, Consoli A, et al. (2016) Semaglutide and Cardiovascular Outcomes in Patients with Type 2 Diabetes. N Engl J Med. 375(19):1834–1844. doi:10.1056/nejmoa1607141
- Marso SP, Daniels GH, Brown-Frandsen K, et al. (2016) Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 2016;375(4): 311–322. doi:10.1056/nejmoa1603827
- CAPRIE Steering Committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996 Nov 16;348(9038):1329-39. doi: 10.1016/s0140-6736(96)09457-3. PMID: 8918275
- Sabatine MS, Giugliano RP, Keech AC, et al. Evolocumab and Clinical Outcomes in Patients with Cardiovascular Disease. N Engl J Med. 2017;376(18):1713-1722. doi:10.1056/nejmoa1615664
- Schwartz GG, Steg PG, Szarek M, et al. (2018) Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med. 379(22): 2097–2107. doi:10.1056/nejmoa1801174
- Eikelboom JW, Connolly SJ, Bosch J, et al. (2017) Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N Engl J Med. 377(14): 1319–1330. doi:10.1056/nejmoa1709118
- Mcmurray JJ, Packer M, Desai AS, et al. (2014) Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med. 371(11) :993–1004. doi:10.1056/nejmoa1409077
- Holman RR, Bethel MA, Mentz RJ, et al. (2017) Effects of Once-Weekly Exenatide on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med. 377(13): 1228–1239. doi:10.1056/nejmoa1612917
- Perkovic V, Jardine MJ, Neal B, et al. (2019) Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N Engl J Med. 380(24): 2295–2306. doi:10.1056/nejmoa1811744
- Mcmurray JJ, Solomon SD, Inzucchi SE, et al. (2019) Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N Engl J Med. 381(21):1995–2008. doi:10.1056/nejmoa1911303
- Packer M, Anker SD, Butler J, et al. (2020) Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 383:1413–1424. DOI: 10.1056/NEJMoa2022190. https://www.nejm.org/doi/full/10.1056/NEJMoa2022190
- Anker SD, Butler J, Filippatos G, et al. (2021) EMPEROR-Preserved Trial Investigators. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N Engl J Med. doi: 10.1056/NEJMoa2107038. Epub ahead of print. PMID: 34449189.
- Mcmurray JJ, Packer M, Desai AS, et al. (2014) Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N Engl J Med. 383. 371(11): 993-1004. doi:10.1056/nejmoa1409077
- McMurray JJV, Wheeler DC, Stefánsson BV, et al. (2021) DAPA-CKD Trial Committees and Investigators. Effects of Dapagliflozin in Patients With Kidney Disease, With and Without Heart Failure. JACC Heart Fail. Aug 5: S2213-1779(21)00334-6. doi: 10.1016/j.jchf.2021.06.017. Epub ahead of print. PMID: 34446370.
- Jackson AM, Jhund PS, Anand IS, et al. (2021) Sacubitril-valsartan as a treatment for apparent resistant hypertension in patients with heart failure and preserved ejection fraction. Eur Heart J. 2021 Aug 15: ehab499. doi: 10.1093/eurheartj/ehab499. Epub ahead of print. PMID: 34392331
- CDC (2017) FastStats - Diabetes. Centers for Disease Control and Prevention. https://www.cdc.gov/nchs/fastats/diabetes.htm. Published May 3, 2017. Accessed June 25, 2020.
- Rowley WR, Bezold C, Arikan Y, Byrne E, Krohe S. (2017) Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul Health Manag. 20(1): 6–12. doi:10.1089/pop.2015.0181
- Huang, E. S., Basu, A., O'grady, M., & Capretta, J. C. (2009). Projecting the Future Diabetes Population Size and Related Costs for the U.S. Diabetes Care. 32(12), 2225-2229. doi:10.2337/dc09-0459
- Riddle MC, Herman WH. The Cost of Diabetes Care—An Elephant in the Room. Diabetes Care. 2018;41(5):929-932. doi:10.2337/dci18-0012
- American Diabetes Association. (2018) “Economic Costs of Diabetes in the US in 2017” Diabetes Care 2018 Mar; dci180007 https://doi.org/10.2337/dci18-0007
- Mata-Cases M, Rodríguez-Sánchez B, Mauricio D, et al. (2020) The Association Between Poor Glycemic Control and Health Care Costs in People With Diabetes: A Population-Based Study. Diabetes Care. 43(4): 751–758. doi:10.2337/dc19-0573
- Hall MJ, Rui P, Schwartzman A. Emergency department visits by patients aged 45 and over with diabetes: United States, 2015. NCHS Data Brief, no 301. Hyattsville, MD: National Center for Health Statistics. 2018
- McEwen LN, Herman WH. Health Care Utilization and Costs of Diabetes. In: Cowie CC, Casagrande SS, Menke A, et al., editors. Diabetes in America. 3rd edition. Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases (US); 2018 Aug. CHAPTER 40. Retrieved June 10, 2020, from: https://www.ncbi.nlm.nih.gov/books/NBK567979/



