Annals of Cardiology
Annals of Cardiology is a peer-reviewed, fully open access, online-only journal that publishes research in therapeutic areas and all disciplines of cardiology. The journal welcomes original research, review articles, educationally valuable case reports, quality improvement projects, images, perspectives, editorials and commentaries related to prevention, diagnosis and treatment of congestive heart failure, congenital heart disease, hypertension, cardiomyopathy, valvular heart disease, arrhythmia and coronary artery disease (please read the subjects covered by the journal below to learn more).
The journal aims to provide a unique platform for medical staff members, young cardiologists and clinicians to learn specifically about rare medical cases and conditions discovered through the latest methods of examination, in addition to the epidemiological, genetic and pathophysiological topics.
Some of the subjects covered include:
- Hypertension
- Vascular disease
- Arrhythmia and electrophysiology
- Cardiac disease
- Heart failure
- Clinical and translational research in cardiology
- Lifestyle and prevention
- Imaging and diagnostic testing
- Transplantation and surgical interventions
- Stroke
- Circulation
- Critical care
Editorial Contact
Kenneth Hall, Managing Editor
Journal Information
ISSN: 2638-1745
Current Volume: Volume 1, Issue 1
Open Access: For all article types
Abstracting and Indexing
Impact metrics
The following citation metrics are produced by abstracting and indexing databases using their respective datasets.
Google Scholar (h5-index and h5-median scores awaiting)
Benefits of publishing with us
Open Access
All articles published by Annals of Cardiology are made freely and permanently accessible online immediately upon publication, without subscription charges or registration barriers.
Article Processing Charges
Annals of Cardiology accomplish global open access. The journal, therefore, levies an affordable article-processing charge of $1495 for each article accepted for publication.
Peer Review — High standard, rigorous peer review
All articles that reach us undergo a double-blind peer review by at least two anonymous reviewers before being considered for publication in the journal.
Post-publication Open Peer Review
Following publication, the peer review comments would be made open for anyone to read. The authors have the option to make the peer review history publicly available after publication.
Research Promotion
Increased credibility and wider dissemination of published articles.
FEATURED ARTICLES
Senior Editors
Yiru Guo
Division of Cardiovascular Medicine
Department of Medicine,
University of Louisville, USA
Govindasamy Ilangovan
Department of Internal Medicine
The Ohio State University, USA
Gyanendra Sharma
Division of Cardiology
Department of Medicine
Augusta University, USA
James V. Cireddu
Case Western Reserve University, USA
Editorial Board
Huan Tao
Department of Medicine
Vanderbilt University, USA
Michael Del Core
Department of Medicine
School of Medicine
Creighton University, USA
Jolanta Vaskelyte
Department of Cardiology
Lithuanian University of Health Sciences, Lithuania
Erika D. Feller
University of Maryland, USA
Afshan B. Hameed
Department of Medicine, Division of Cardiology
University of California, Irvine, USA
Xudong Liao
Case Western Reserve University, USA
Guidelines for Submission
Manuscript Preparation
Submission and Components
Submissions to Rivera journals should include the following:
- Cover Letter
- Manuscript
- Figures and Tables
- Supplemental Materials
Authors are encouraged to submit all the components as 'zip file' while submitting on our online system or via email as an attachment.
Cover Letter should:
- briefly summarize or provide an outline of your manuscript, and why it is a worthy contribution to the concerned journal;
- specify the Rivera journal that your manuscript best pertains to;
- indicate, if applicable, that it is submitted as a part of Special Issue;
- specify the manuscript type (original research, review, etc.)
- detail any previous interaction(s) with Rivera Publications (previously submitted)
- specify all authors' information, including affiliation
- include acknowledgments and funding information (if applicable) and any competing interests
Authors
All persons who qualify for authorship should be listed as authors. However, the corresponding author must ensure that each author listed has substantially contributed or participated sufficiently in the work and is responsible for that particular portion of the manuscript. However, people who do not qualify for authorship should be listed in acknowledgments.
One author (corresponding author) should be listed with an asterisk and should provide his or her email address. For the remaining authors, if applicable, following information should be included:
- First name and last name
- Complete affiliation, along with the country
- Email address (optional, but mandatory for the corresponding author)
For example:
Robert Ludwig1
Yale University, New Haven, Connecticut, USA
Acknowledgements
This should include all the people who have contributed toward the work in one way or the other. However, authors are required to ensure that people acknowledged should agree to being so named.
Funding Information
List all the sources of funding, including relevant research grant numbers, as applicable. Also, authors are encouraged to list all the contributing authors associated with specific funding, if applicable.
Conflict of interest
The corresponding author is required to provide a statement of conflict of interest on behalf of all the authors. For further information, please refer to our Conflict of Interest Policy page.
Optional information
While we are not obligated to use these or recommend to the concerned Editor(s), we do encourage authors to provide names and contact information of 2-4 external reviewers and, if applicable, 1-2 opposed reviewers.
Manuscript Layout
The word count for original research is 3500–4000 words and up to 5500 words for studies involving meta-analysis. Authors are encouraged to employ a standard and concise writing style. If you are not a native English speaker, we encourage you to utilize our language editing services-or ask a native English speaking colleague for assistance.
All manuscript submissions can have the following sections:
- Title
- Abstract
- Keywords
- Main Text
- Introduction
- Materials and methods
- Results
- Discussion
- Conclusion(s)
- References
Title
The title should not exceed 200 characters and set in title case. The title should be concise, specific, and easily comprehensible to readers.
Abstract
The abstract should not exceed 300 words and should be unstructured (without sub-heading such as objective, methodology, results, discussion, etc.). It should provide a clear description of the objective(s) of the study, demonstrate the methodology used, and summarize the study's prime conclusion(s). At the end, a statement regarding the study's significance to a potentially wider audience should be included.
Keywords
Authors can provide 4-6 keywords. The first letter of each keyword should be upper case, and keywords should be separated by a semicolon (;).
References
Published work along with any citable items should be cited in the reference list. While we follow very stringent reference formats, authors need not to spend time formatting their reference. They can submit the manuscripts formatted in any reference style (style will be formatted once the manuscript is accepted for publication), but it is preferable that they adhere to the journal format.
For Health and Medicine
Rivera uses AMA style. Items are listed numerically in the order they are cited in the text.
Example journal article (2-6 authors): Salwachter AR, Freischlag JA, Sawyer RG, Sanfey HA. The training needs and priorities of male and female surgeons and their trainees. J Am Coll Surg. 2005; 201: 199-205.
Example journal article (more than 6 authors): Fukushima H, Cureoglu S, Schachern P, et al. Cochlear changes in patients with type 1 diabetes mellitus. Otolaryngol Head Neck Surg. 2005; 133: 100-6.
Example book: Modlin J, Jenkins P. Decision Analysis in Planning for a Polio Outbreak in the United States. San Francisco, CA: Pediatric Academic Societies; 2004.
Example book chapter: Solensky R. Drug allergy: desensitization and treatment of reactions to antibiotics and aspirin. In: Lockey P, ed. Allergens and Allergen Immunotherapy. 3rd ed. New York, NY: Marcel Dekker; 2004:585-606.
Example online article: Wolf W. State's mail-order drug plan launched. Minneapolis Star Tribune. May 14, 2004:1B.
Example article from any database: Calhoun D, Trimarco T, Meek R, Locasto D. Distinguishing diabetes: Differentiate between type 1 & type 2 DM. JEMS [serial online]. November 2011; 36(11):32-48. Available from: CINAHL Plus with Full Text, Ipswich, MA. Accessed February 2, 2012.
In-text citation: For referencing an article, a number is used. This is different from in-text citations in AMA—author's last name is not used. The order of numbering will be contingent on the order in which you use that reference within your paper. For example, the first article referenced will be given number one in superscript (1) followed by second and third articles as 2,3. In references section, the articles should appear numerically in the order they are cited within the text.
Figures and Tables
Figures and tables should be included in the main text (manuscript) to aid in the review process. However, for larger files (size exceeding 10 Mb) must always be submitted separately (should be properly mentioned in the main text, wherever applicable).
Figure captions and legends
Figure files should be included in the main document, and not as supplemental materials. Figure caption should be preceded by the figure, while figure legends should immediately follow the figure. Figure captions should be concise (not to exceed 18 words) and set in bold type. All figures should be numbered in sequence, using Arabic numerals, for example, Figure 1, Figure 2, etc.
Table captions and legends
Tables should be cited in ascending numeric order. Each table should be preceded by a table caption (brief and specific; not to exceed 18 words), and immediately followed by table legends, if applicable, used to explain abbreviations and other supporting information about the data. Larger tables, however, can be submitted as supplemental materials.
Review Comments and Revision
Submission Instructions
While submitting a revised manuscript, the authors should include the following:
Revised manuscript (clean copy): Prepare a clean copy of your revised manuscript that does not show track changes. Rename this file as "Main Document".
Revised manuscript (marked-up copy): Include a copy of your manuscript file showing the changes you have made (track changes). Rename this file as "Manuscript with Track Changes".
Response to reviewers: Address the specific points made by each Reviewer and/or Editor. Include your responses to all the reviewers' and editor's comments and list the changes you have made to the manuscript. Rename this file as "Response to Reviewers".
Supplementary Information
Information integral to the comprehensive understanding of the manuscript, but is either too large to be included in the main document or due to any other reason, should be submitted as support materials, such as 3-D visualizations, interactive graphics, large tables and/or figures, and so on. However, authors should note that normal figures and tables should not be included under supplemental materials.
Ethical Guidelines
For manuscripts reporting medical studies that involve human or animal subjects, ethics committee that approved the study must be identified in the manuscript. For studies involving human subjects, all work must conform to the recognized standards as per the "Declaration of Helsinki". In case of any experiments involving animals, authors must provide a declaration that all measures were taken to avoid animal suffering at each stage and also must furnish a detailed description of the procedures used.
Patient Consent
In manuscripts reporting patient cases, patient anonymity must be preserved. Case reports submitted to Rivera Publications should conform to the International Committee of Medical Journal Editors' (ICMJE) recommendations. Patient privacy should be taken care of, and personally identifiable information should not be revealed without informed consent. If informed consent has been obtained, the details must be mentioned in the manuscript.
For live patients, signed consent is mandatory if the authors wish to reveal the patient's identity. In case of deceased patients, consent must be taken from patient's next of kin. If patient's consent was not obtained, patient's details should be anonymized as much as possible. Patient's photographs need to be cropped sufficiently to prevent revelation of identity.
Authors are not required to submit the copy of the patient's consent while submitting their manuscript for consideration in Rivera Publications. However, they should confirm in the Cover Letter that the patient's consent has been obtained. In certain instances, the Editorial Office might request the authors to provide a copy of the same.
Articles in Press
Volume 2, Issue 1
Hassan Saleh, Kush Patel, Nicholas Pavlatos, Emily Converse, Hesham Affify, Thomas Beyerle, Robert Whitford, Ravi Sharma, Sohail Ikram, Marcel Letorneau, Naresh Solankhi
Research: Ann Cardiol 2:1: 1
Aug 25, 2025 DOI: 10.31532/AnnCardiol.2.1.001
Volume 1, Issue 1
Mohsin. Ibrahim, and Ahmed. M. Habeeb
Case report: Ann Cardiol 1(1): 1
May 03, 2019 DOI: 10.31532/AnnCardiol.1.1.001
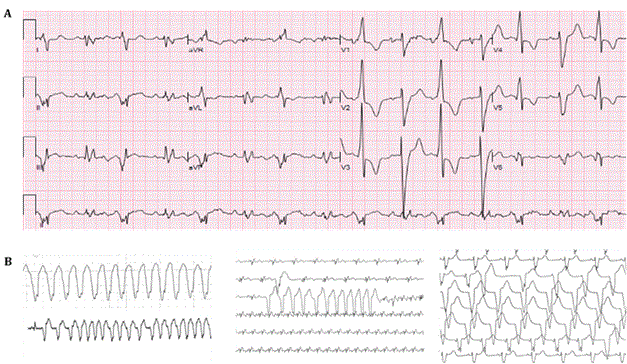
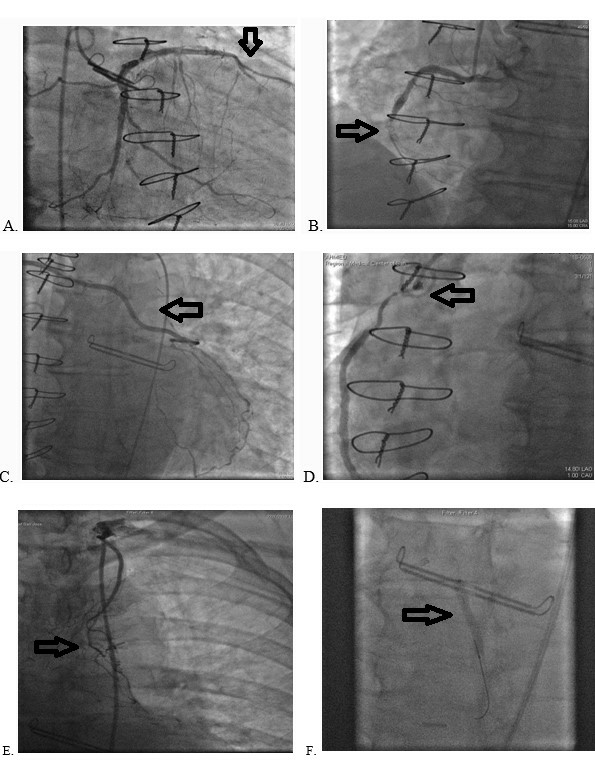
Multiple Arrhythmias in COVID-19 Patients: A Case Series
Savalan Babapoor-Farrokhran, Zachary Port, Deanna Gill, Zaid Ammari, Sumeet Mainigi, and Aman Amanullah
Case report: Ann Cardiol 1(1): 2
Oct 20, 2020 DOI: 10.31532/AnnCardiol.1.1.002
Cardioprotection: Challenges and Perspectives
Emanuel Tenório Paulino
Commentary: Ann Cardiol 1:1: 3
Jun 09, 2025 DOI: 10.31532/AnnCardiol.1.1.003

J-Gate
J-Gate is bibliographic database to access global e-journal literature – indexing over 71 million journal articles, updated daily.
© 2026. Rivera Publications, Inc. All rights reserved.