Home / Journal / Oral Health and Dental Studies
Photo-cross-linked Antibacterial Zein Nanofibers Fabricated by Reactive Electrospinning and its Effects against Streptococcus mutans
Zein Nanofibers Photo-cross-linking Solvent resistance Antimicrobial
Jian-Feng Zhang, Yapin Wang, Sumei Liao, Thomas Lallier, Zezhang T. Wen, and Xiaoming Xu
DOI: 10.31532/OralHealthDentStud.1.1.001 13 Sep 2017
Abstract
Native zein electrospun nanofibers have shown poor solvent resistance and low mechanical strength. Compared to other toxic cross-linkers, a safer method of stabilizing zein based fibers while retaining or with improved mechanical strength is needed to convert these materials for biomedical applications where culture media or body fluids may be present. We report here a method of fabricating non-toxic zein nanofibers using reactive electrospinning coupled with in situ photo-cross-linking. The cross-linked zein nanofibers exhibited significantly improved mechanical strength and sustained morphology against water and aqueous ethanol solution. This process doesn’t require additional conventional cross-linking agents to form cross-linking network, which is advantageous for biomedical application. Antimicrobial monomer with photo-reactive moiety was coupled with methacrylate zein nanofibers and showed strong inhibitory activity against cariogenic Streptococcus mutans. Cytotoxicity test with human gingival fibroblasts revealed high biocompatibility.
Keywords
Zein; Nanofibers; Photo-cross-linking; Solvent resistance; Antimicrobial
Introduction
Zein, a hydrophobic prolamine maize protein, has received considerable attention for a variety of industrial and food uses due to its renewable resource origination and biodegradation capability. It is non-allergenic and generally recognized as safe for biomedical application and is approved by the U.S. Food and Drug Administration. Chemically, a high proportion of nonpolar groups on amphiphilic zein enable it soluble in organic solvents such as dimethylformamide (DMF), dimethyl sulfoxide, and ethanol aqueous solution, which is more environmentally benign.[1] Appropriate zein concentrations in 50 – 95% ethanol aqueous solution generate necessary chain entanglement for micro- and nanofibers formation by electrospinning. Water, however, can dramatically alter nanofibers’ surface topography. Reactive amino and hydroxyl groups on zein lead to the possibility of several chemical modifications and subsequently alter its structure and functionality, including zein nanofibers’ solvents resistance.[2–7] Cross-linking has been proven one of the most effective methods in stabilizing fibrous structure and improving mechanical properties. A variety of chemical cross-linking agents including formaldehyde,[2] glutaraldehyde,[3] citric acid,[4] hexamethylene diisocyanate (HDI),[5] methylenediphenyl diisocanate (MDI),[6] and glyoxal[7] have been explored to cross-link zein nanofibers. However, in these systems, either cross-linkers are toxic (e.g., formaldehyde, glutaraldehyde) or the cross-linking reaction takes extended period of time or requires high temperature to complete. For instance, the cross-linking by HDI or MDI in tetrahydrofuran requires about 24 hours,[5,6] and citric acid takes more than 2 h at high temperature e.g., 130°C.[4] A quicker and safer cross-linking process is advantageous over traditional chemical cross-linkers to enable zein as emerging nanomaterials, in particular for biomedical applications.
Antibacterial materials indicate their significant promises for a variety of potential medical applications.[8] For example, antibacterial polymeric nanofibers as wound dressing scaffolds with releasable antibacterial agents such as nanoparticles,[9] ciprofloxacin,[10] streptomycin sulfate,[11] have been developed. Poly (lactic-co-glycolic acid) nanofibers with bioactive glass showed antibacterial activity against oral pathogens.[12] Imparting efficient and long-last antibacterial activity onto zein nanofibers is expected to expand their applications.
To our best knowledge, there is no report on photo-cross-linking of zein in any forms including bulk materials, films and fibers. Herein we report a process of fabricating cross-linked zein nanofibers by reactive electrospinning with immobilized antibacterial function. We believe this could be served as a model technique in developing nanofibrous materials with long-last antibacterial activity for a wide variety of applications including oral healthcare, drug delivery, medical devices, wound healing, infection control, and tissue engineering.[13,14]
Materials and Methods
Materials Alpha-zein with 3.4% moisture with molecular weight of 22–24 kD was purchased from Sigma-Aldrich (St. Louis, MO). Denatured ethyl ethanol was purchased from Acros Organics (Geel, Belgium). Anhydrous DMF, potassium carbonate, phenyl-bis (2,4,6-trimethylbenzoyl)-phosphine oxide (PO), and methacrylate anhydride were purchased from Sigma-Aldrich (St. Louis, MO). All solvents were used as received without further purification.
Synthesis of Methacrylated Zein Vacuum dried zein (3.0 g, 50°C for 24 h) was dissolved in 30 mL anhydrous DMF, and 3.0 g K2CO3 was also added to the solution and cooled to 0°C. Methacrylic anhydride (4.0 mL) was added drop wise to the solution and stirred at 0°C for 30 min. The solution was let to warm up to room temperature in about 30 min, and the reaction was allowed to proceed for 48 h shielding from direct light to prevent polymerization. Then the reaction mixture was washed with excess amount of deionized water and filtered to remove K2CO3. The pale-yellowish residue was dissolved in large amount of 70% ethanol aqueous solution (ca. 100 mL) and precipitated with ethyl acetate, and repeated three times to remove DMF and byproducts of methacrylate acid. The precipitates were further dried under vacuum at room temperature to minimize solvent residues. The yield is 90.6%.
Electrospinning of Zein Nanofibers An electrospinning device with spinneret consisted of a heated, pneumatic assisted stainless steel tubing assembly was used in the current work.[15] Native zein or metharylic zein in 80% ethanol solution was infused by a syringe pump (KDS 100, KD Scientific Inc., Holliston, MA) with a flow rate of 0.3 mL/h to the spinneret with 14 cm distance from the front edge of the collecting drum. A high voltage power supply (Gamma High Voltage Research, Inc., Ormond Beach, FL) was used to provide -30 kV to the rotating drum. Electrospinning jet was irradiated by an UV lamp at 140 mW/cm2. After stopping the syringe pump, additional 5 min UV irradiation was performed to allow sufficient crosslinking of the nanofibers.
Characterization Native zein and methacrylic zein were characterized by Nexus 670 FT-IR Spectrometer (Thermo-Nicolet, Madison, WI) equipped with NIR Updrift ATR accessory (Pike Technologies, Madison, WI) in a wave number range of 6400–5600 cm-1. Particle size of 0.1% native zein (w/v) and methacrylic zein dissolved in 80% ethanol solution was measured by Microtrac Nanotrac 250 (Montgomeryville, PA). Morphology and diameter of electrospun zein nanofibers were analyzed by a scanning electronic microscopy (Hitachi S-2700, Hitachi-High Technologies America, Pleasanton, CA).
Antibacterial Assay Antibacterial monomer with photo-reactive moiety was synthesized in a procedure reported previously.[16] The monomer with three concentrations as well as 1% photo-initiator of PO was dissolved in the electrospinning solution containing methacrylic zein. After UV sterilization, the weighed electrospun nanofibers (about 5 mg) were properly laid at the bottom of a glass cultural tube. For antibacterial assay, overnight broth culture of Streptococcus mutans, a key etiological agent of human dental caries, was transferred to fresh, pre-warmed brain heart infusion (BHI, Becton, Dickinson and Company, MD) and allowed to continue to grow in an aerobic chamber with 5% CO2 at 37oC.[17,18] When reaching mid-exponential phase (OD600nm ~0.5), the cultures were diluted 1:100 with fresh medium and aliquots (5 mL) were applied to the glass cultural tubes with zein nanofibers, and bacterial growth at 37oC was monitored every 30 min continuously for 10 h by using a Spectronic 20D+ (Thermo Scientific, Pittsburg, PA).
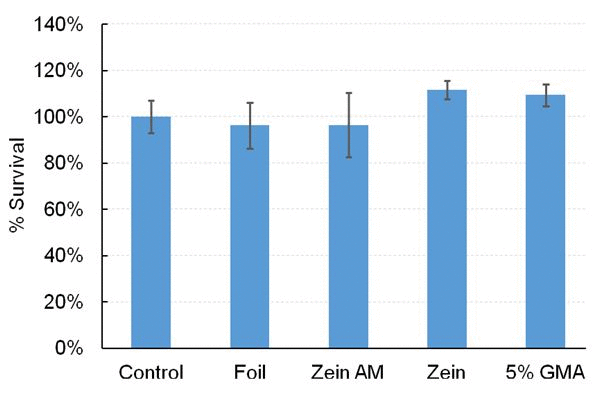
Cytotoxicity Test Human gingival fibroblasts were obtained from extracted molars from patients with healthy gingival following informed consent as prescribed in an approved IRB protocol. Gingival fibroblasts were maintained in MEMα containing 10% fetal calf serum (FCS) and 200 units/mL penicillin and 200 mg/mL streptomycin. Cells were grown in 24-well plates for 24 h prior to fiber on the aluminum foil. The growth media containing 0.1% dimethylsulfoxide (DMSO) were supplemented with aluminum foil alone, m-zein-AM2 nanofibers, methacrylated zein, 5% GMA and AM2 on aluminum foil supports were added to cells for 24 h. In addition, growth media supplemented with various concentration of AM2 monomer (10-3 M, 10-4 M, 10-5 M, 10-6 M and 10-7 M) were added to the cells for 24 h. MEMα served as a control for cytotoxicity. Cell survival was visualized using a fluorescent esterase substrate (Calcein-AM) and a Nikon TE2000 inverted fluorescent microscope. Cell survival was quantified using a BioTek Synergy 2 fluorescent multi-well plate reader.
Results and Discussion
Methacrylated zein In native zein, hydrogen bonds, van der Waals forces and ionic interactions are major inter- and intramolecular forces. Due to longer intermolecular spaces, forces between polypeptide chains are much weaker than those within the molecules, which are more prone to break or weaken in aqueous solutions.[4] Although zein is hydrophobic naturally,[19] the weak intermolecular forces more prominently at nanoscale are inadequate to hold polypeptide tightly in aqueous solutions. The strong intramolecular interactions are mainly the contribution of the high content of non-ionizable amino acid residues, such as glutamine, leucine, alanine, proline, serine and phenylalanine, which are relatively intact in aqueous solutions.[20]
At macro scale, resultant zein nanofibers are an integration of these polypeptides chains. Both inter- and intra-molecular forces are governing factors influencing solvent resistance of zein nanofibers. Therefore, in order to improve fibrous structure integrity against aqueous solution, it’s necessary to alter zein’s functional groups to strengthen inter-molecular forces through appropriate modifications. It has been well documented that glutamine, histidine, cysteine, serine, threonine, arginine, tyrosine, and glutamic acid are possible amino acids in α-zein with active end or side groups capable of reacting with other reactive groups.[21]
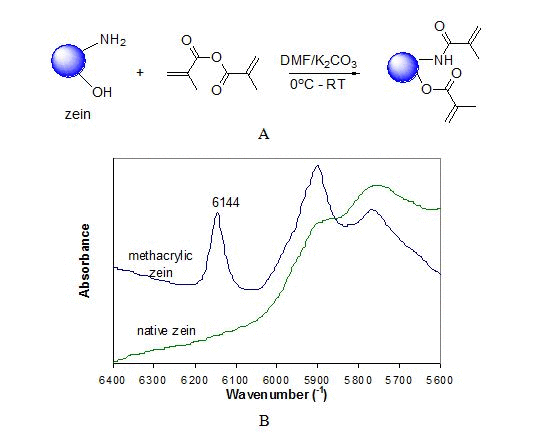
In preparation of photo-reactive zein, with the assistance of K2CO3 the well dispersed zein in DMF with unfolded appropriate polypeptides allows sufficient methacrylation on appropriate functional groups as illustrated in Figure 1A. Methacrylic groups were successfully anchored on zein as evidenced by prominent vinyl C=C bonds (6144 cm-1) [22] shown on the FT-NIR spectra in Figure 1B. The introduction of methacrylic groups on zein (denoted as m-zein) makes it more hydrophobic than native zein which would change its dispersion status in ethanol aqueous solution largely due to its isoelectric point. Orientated out layer with rich hydrophilic moiety in native zein aggregates with less than 90% ethanol was suggested.[23] With reduced number of hydrophilic groups on outside layer of globules-like structure, the surface characteristic and polypeptide folding behavior are therefore altered. It seems that m-zein aggregates tend to attract each other forming large globules size around 2 µm in comparison to 600 nm of native zein in 80% ethanol solution evidenced by the particle size analysis.
Figure 1. (A) Synthesis scheme for photo-cross-linkable zein. (B) Near FT-IR spectra of methacrylic zein and native zein.
Generation of methylated zein nanofibers by electrospinning Appropriate zein concentration in a range of 18–35%, ethanol aqueous solution concentration of 50–80% and electrospinning solution viscosity play key roles in round or ribbon fibers formation and diameter control.[7, 24] Readily dissolvable m-zein in ethanol aqueous solution with 1% PO as photo-initiator facilitates electrospinning by an environmentally friendly process. Smooth electrospun native zein nanofibers have an average diameter of 310 ± 97 nm (Figure 2A). It has been demonstrated that native zein nanofibers have poor solvents resistance,[2–6] even water contact can dramatically deteriorate fiber integrity as shown in Figure 2B due to weak intermolecular forces. The electrospun zein nanofibers can be readily dissolved in ethanol aqueous solution (Figure 2C). At the same concentration, m-zein produces uniform nanofibers with an average diameter of 298 ± 102 nm (Figure 2D), which is similar with native zein nanofibers. Initial large particle size of m-zein seems not induce thick fibers or particles compared to native zein which implies that the micelles may have loosely packed structure and similar stretching behavior during electrospinning. Without cross-linking, the m-zein nanofibers remain susceptible to water (Figure 2E) and ethanol aqueous solution immersion (Figure 2F). Upon in situ UV irradiation during electrospinning, the produced cross-linked m-zein nanofibers remain similar structural morphology (348 ± 146 nm, Figure 2G) with electrospun native zein or m-zein without UV irradiation. The UV cross-linked zein nanofibers exhibited stable morphology against water (344 ± 97 nm, Figure 2H), and ethanol aqueous solution (336 ± 152 nm, Figure 2I), which demonstrates an exceptional improvement in zein nanofibers solvent resistance.
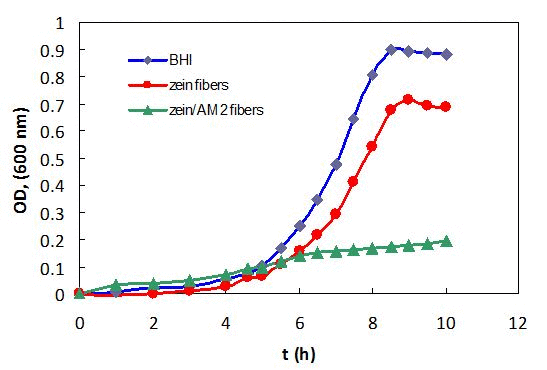
The zein nanofibers displayed strong antibacterial property Antibacterial monomer (AM2) with methacylate moiety has been proven to inhibit the growth of certain oral pathogens.[16] Upon UV irradiation, the AM2 is capable of covalently bonding to methacylate zein nanofibers adding antibacterial function (Figure 3). The m-zein with 10% of AM2 (m-zein basis) was electrospun to smooth nanofibers upon UV irradiation (Figure 4A) and these nanofibers retained smooth fibrous structure after 75% ethanol solution immersion for 3 days (Figure 4B). When analyzed using broth cultures, S. mutans displayed little or no growth in the presence of cross-linked antibacterial zein nanofibers, indicative of strong inhibitory effects of the antibacterial monomer AM2 containing nanofibers (Figure 5). Interestingly, relative to those in plain BHI, cultures received zein nanofibers with no AM2 also displayed some reduction in growth rate and culture density, which suggests that zein itself may also possess some weak antibacterial activity against S. mutans growth.
Figure 2. SEM of electrospun zein nanofibers. Native zein: (A) As electrospun. (B) After water immersion. (C) After 75% ethanol immersion; methacrylic zein: (D) As electrospun (298±102 nm). (E) After water immersion. (F) After 75% ethanol immersion; UV cross-linked methacrylic zein (348 ± 146 nm): (G) As electrospun. (H) After water immersion. (I) After 75% ethanol immersion (336 ± 152 nm) (all magnification: ×5k).
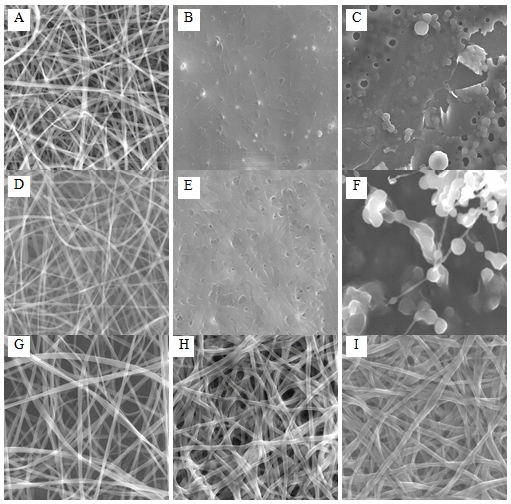
Figure 3. (A) Schematic reaction of antimicrobial monomer (AM2) cross-linked with methacrylated zein during reactive electrospinning. (B) FT-IR spectra of zein and antibacterial zein nanofibers.
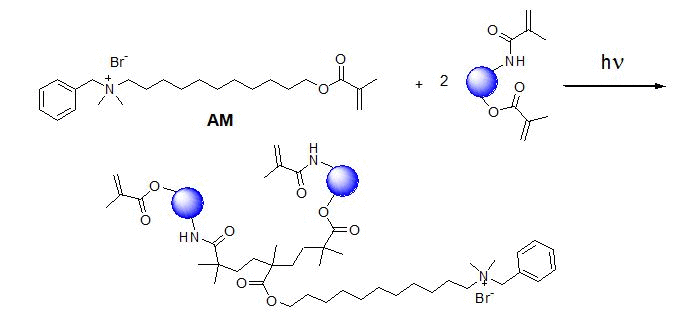
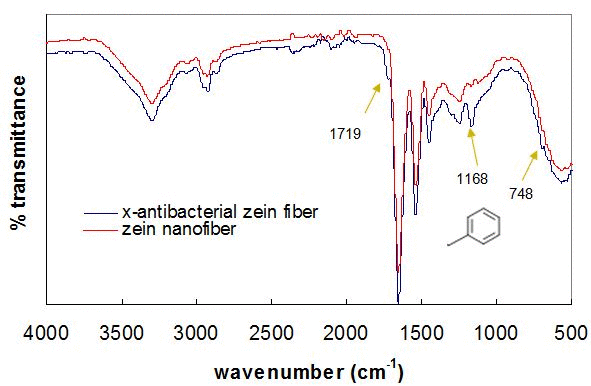
Figure 4. Electrospun from 30% methacrylated zein in 80% ethanol with 3% antimicrobial monomer (AM2) and irradiated by UV at 140 mW/cm2. (A) As electrospun and cross-linked. (B) Cross-linked fibers after 3-day immersion in 75% ethanol aqueous solution.
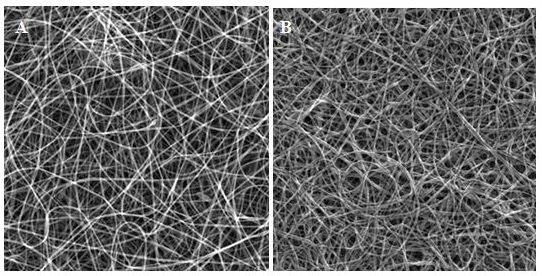
Figure 5. Effect of antibacterial zein nanofibers on S. mutans growth during growth in BHI with zein nanofibers without monomer AM2 and plain BHI serving as a control. Data are representatives of three separate experiments.
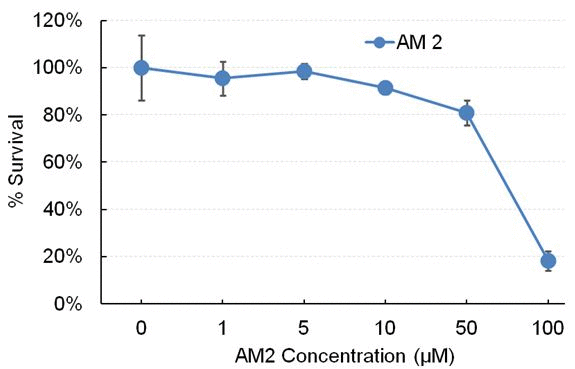
Cytotoxicity It is important that any antibacterial component of new dental or biomaterials show sufficiently low cytotoxicity to healthy cells in order to make it a clinically viable product. In an earlier study, we have demonstrated that monomer AM2 has good biocompatibility at 10-4 M concentration (the different concentrations tested in the Bioscreen analysis, inhibit zoom and bio film).[25] The cytotoxicity of AM2 displayed up to 80% cell survival except at concentration of 10-2 M. In addition, m-zein with 10% AM2 nanofibers displayed low cytotoxicity as shown in Figure 6 A and B.
Figure 6. (A) Cytotoxicity of antibacterial monomer AM2. (B) Fabricated crosslinked methacrylated zein nanofibers with AM2.
Conclusion
The derivative of zein with photosensitive methacrylate moiety provides a facile route for photo initiated cross-linking avoiding incorporation of toxic chemical cross-linking agents. The cross-linked zein nanofibers fabricated by the reactive electrospinning technique coupled with in situ UV irradiation retained fibrous architecture in aqueous environment. The chemically immobilized antibacterial monomers on zein nanofibers showed strong inhibition against S. mutans while maintain high biocompatibility. This dynamic process opens up new applications for zein particularly in dental and biomedical areas where antibacterial function is normally required.
Acknowledgement
The authors appreciate financial support from the Joe W. and Dorothy Dorsett Brown Foundation, NIH/NIDCR grant R01DE19203 to X. Xu and NIH/NIDCR grant 5R01DE19452 to Z. T. Wen.
References
- Pelosi LF. Crosslinking processes/agents for zein. 1997: WO/1996/010582. .
- Selling GW, Woods KK, Biswas A. Electrospinning formaldehyde-crosslinked zein solutions. Polym Int. 2011; 60(4): 537–542.
- Selling GW, Woods KK, Sessa D, Biswas A. Electrospun zein fibers using glutaraldehyde as the crosslinking reagent: effect of time and temperature. Macromol Chem Phys. 2008; 209(10): 1003–1011.
- Xu W, Karst D, Yang W, Yang Y. Novel zein-based electrospun fibers with the water stability and strength necessary for various applications. Polym Int. 2008; 57(10): 1110-1117.
- Yao C, Li X, Song T. Electrospinning and crosslinking of zein nanofiber mats. J Appl Polym Sci. 2007; 103(1): 380–385.
- Yao X, Li X, Song T. Fabrication of zein/hyaluronic acid fibrous membranes by electrospinning. J Biomater Sci Polym E. 2007; 18(6): 731–742.
- Suwantong O, Pavasant P, Supaphol P. Electrospun zein fibrous membranes using glyoxal as cross-linking agent: preparation, characterization and potential for use in biomedical applications. Chiang Mai J Sci. 2011; 38: 56–70.
- Charernsriwilaiwat N, Rojanarata T, Ngawhirunpat T, Opanasopit P. Electrospun chitosan/polyvinyl alcohol nanofibre mats for wound healing. Int Wound J. 2014; 11(2): 215–222.
- Tian L, Wang P, Zhao Z, Li J. Antimicrobial activity of electrospun poly(butylenes succinate) fiber mats containing pvp-capped silver nanoparticles. Appl Biochem Biotechnol. 2013; 171(7): 1890–1899.
- Unnithan AR, Barakat NAM, Pichiah PBT, Gannasekaran G, Nirmala R, Cha YS, et al. Wound-dressing materials with antibacterial activity from electrospun polyurethane-dextran nanofiber mats containing ciprofloxacin HCl. Carbohydr. Polym. 2012; 90(4): 1786–1793.
- Hild N, Tawakoli PN, Halter JG, Sauer B, Buchalla W, Stark WJ, et al. pH-dependent antibacterial effects on oral microorganisms through pure PLGA implants and composites with nanosized bioactive glass. Acta Biomaterialia. 2013; 9(11): 9118–9125.
- Zhang Y, Cui L, Chen Y, Zhang H, Zhong J, et al. Zein-based nanofibres for drug delivery: classes and current applications. Curr Pharm Des. 2015; 21(22): 3199–3207.
- Thakur V.K, Thakur M.K. Zein - Prolamine isolated from corn gluten meal. Handbook of Polymers for Pharmaceutical Technologies. USA, NJ: John Wiley & Sons Inc.; 2016: 9.3.
- Xu X, Zhang JF, Fan Y. Fabrication of cross-linked polyethyleneimine microfibers by reactive electrospinning with in situ photo-cross-linking by uv radiation. Biomacromolecules. 2010; 11(9): 2283–2289.
- Xu X, Wang YP, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. J Biomed Mater Res B: Appl Biomater. 2012; 100B: 1151–1162.
- Wen ZT, Yates D, Ahn SJ, Burne RA. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC microbiol. 2010; 10: 111.
- Unnithan AR, Gananasekaran G, Sathishkumar Y, Lee YS, Kim CS. Electrospun antibacterial polyurethane–cellulose acetate–zein composite mats for wound dressing. Carbohydr Polym. 2014; 102: 884–892.
- Bitoun JP, Nguyen AH, Fan Y, Burne RA, Wen ZT. Transcriptional repressor Rex is involved in regulation of oxidative stress response and biofilm formation by Streptococcus mutans. FEMS Microbiol Lett. 2011; 320(2): 110.
- Wolp WJ, Lawton JW. Isolation and characterization of zein from corn distillers' grains and related fractions. Cereal Chem. 1997; 74: 530–536.
- Paulis JW, James C, Wall JS. Comparison of glutelin proteins in normal and high-lysine corn endosperms J Agric Food Chem. 1969; 17(6): 1301–1305.
- Wang Y, Chen L. Electrospinning of prolamin proteins in acetic acid: the effects of protein conformation and aggregation in solution. Macromol Mater Eng. 2012; 297(9): 902–913.
- Xu X, Ling L, Wang R, Burgess JO. Formulation and characterization of a novel fluoride-releasing dental composite. Dent Mater. 2006; 22(11): 1014–1023.
- Kim S. Xu J. Aggregate formation of zein and its structural inversion in aqueous ethanol. J Cereal Sci. 2008; 47(1): 1–5.
- Miyoshi T, Toyohara K, Minematsu H. Preparation of ultrafine fibrous zein membranes via electrospinning. Polym Inter. 2005, 54(8): 1187–1190.
- Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites J Biomed Mater Res B Appl Biomater. 2012, 100B (4): 1151–1162.



