Home / Journal / Physical Medicine and Rehabilitation
Use of 4-aminopyridine for Motor Weakness Due to Charcot- Marie - Tooth Hereditary Motor Sensory Neuropathy
charcot-marie tooth hereditary sensory motor neuropathy 4-aminopyridine demyelination inflammatory neuropathies rehabilitation
Jay M. Meythaler and Jean Peduzzi-Nelson
DOI: https://doi.org/10.31532/PhysMedRehabil.1.1.003 25 Nov 2017
Abstract
Presented is the first case in the literature of orally delivered 4-aminopyridine to improve motor function in a patient with long standing Charcot-Marie-Tooth, Hereditary Motor and Sensory Neuropathy (HMSN). The patient is a 63-year-old male with a long-standing history of motor weakness and pain from HMSN. He has been treated for several years for both pain and weakness. He initially had an intrathecal pump placed in 1989 for delivery of morphine for neurogenic pain. The disease had progressed to the point where he walked with a cane on level surfaces and had developed neuropathic foot ulcers. The patient was started on 4-aminopyridine (4-AP) at 5 mg. per day and increased over two weeks to a dose of 30 mg. per day (10 mg. tid). Motor strength on a 0–5 ASIA motor scale increased in the lower limbs from 3.6 to 4.2 and in the upper limbs from 3.9 to 4.5 and was consistently maintained over 12 months. The patient reported considerable increases in walking endurance, decreased falls and no longer uses a cane on level surfaces for ambulation. There was a mild decrease in the perception of pain as measured by the Visual Analog scale from 4 to 3 in the feet and from 7 to 5 in the hands with no other change in pain medications. This case report indicates that 4-aminopyridine may be effective in improving the motor function of HMSN patients. The mechanism of action of 4-AP is theorized to be improved nerve conduction by restoration of the action potential in demyelinated axons. Because the peripheral nervous system has less of a barrier to systemic delivery drug delivery, it makes sense 4-AP may be more effective in peripheral demyelinating diseases than in central demyelinating diseases of the nervous system.
Keywords
Charcot-marie tooth, hereditary sensory motor neuropathy, 4-aminopyridine, demyelination, inflammatory neuropathies, rehabilitation
Introduction
Charcot-Marie-Tooth (CMT) disease is a genetically heterogeneous group of disorders that is characterized by wasting and weakness of distal limb muscles, particularly the peroneal muscles, with or without distal sensory loss.1 The disease onset usually occurs during the first decades of life and course is slowly progressive. It may lead to profound motor impairments as well as painful dysaesthesias and paraesthesias.
Nerve conduction studies demonstrate slowing of nerve conduction velocities consistent with a demyelinating process. Treatment has generally been supportive, consisting of pain medications for painful dysaesthesias, and prescription of orthotics and/or assistive devices for gait.2,3 Patients are usually schooled in energy conservation techniques and joint protection. While there has been considerable progress in defining the genetic and molecular basis for the disease, there has been very little in any treatment to improve motor function.
4-Aminopyridine (4-AP) 4-Aminopyridine, a specific blocker of voltage-dependent, fast activating neuronal potassium channels, has been reported to reduce spasticity and improve motor and sensory function in animal models and in patients with multiple sclerosis (MS)4–6, spinal cord injury (SCI)7–12, and Guillain-Barré, Syndrome (GBS). Animal and human studies indicate that 4-AP may act through restoration of action potential conduction in damaged, poorly myelinated nerve fibers and may also directly enhance synaptic transmission.7–11,13 Preliminary clinical studies have demonstrated that 4-AP is associated with decreased pain and/or improved motor function.4-6,12
Reported is the first clinical use of 4-AP as a retrospective case report in which compounded 4-AP was utilized to improve motor function and endurance in a patient with progressive CMT.
Case Report
The patient is a 63-year-old white male with an over thirty-year history of progressive lower extremity (LE) weakness and numbness diagnosed as CMT disease classified as CMT 1a several years ago. Previous nerve conduction studies performed years earlier had no peroneal motor or sensory responses, absent tibial sensory nerve responses and slowed tibial motor nerve conductions with reduced amplitudes. He had been prescribed bilateral ankle foot orthotics over 10 years previously. Approximately twenty years ago he began to develop severe dysaesthesias in both his hands and feet, which eventually required implantation of a subcutaneously placed pump for the delivery of intrathecal morphine eleven years ago.
Over the last six years he has developed progressive weakness and a loss in his endurance. Because his form of CMT is predominantly demyelinating, after a discussion with the patient where he requested to know of any available medications that could be useful in CMT we decided to implement a trial with open label 4-AP to improve motor strength and endurance. The dosage was started at 5 mg per day and increased by 5 mg every two days until the dosage reached a maximum of 30 mg. per day (10mg tid). The following parameters were followed: Motor score: Monthly The motor score measures the strength of key muscles bilaterally on a 0–5 scale and is closely related to the ASIA motor scoring technique. Use of these standardized measures has been endorsed throughout the rehabilitation community. This scale grades motor strength of selected muscle groups as follows: 0 - Absent (total paralysis) 1 - Trace (palpable or visible contraction producing little or no movement around joint) 2 - Poor (active movement through much or all of the normal range of motion, with gravity eliminated - where relevant) 3 - Fair (active movement through full range of motion against gravity) 4 - Good (active movement against some resistance) 5 - Normal (active movement against full resistance).
The patient also was rated on the visual analog scale for pain in the hands and feet and he received electrodiagnostic testing. Safety data were monitored including an electrocardiogram, and laboratory testing before starting the medication and after two months of treatment.
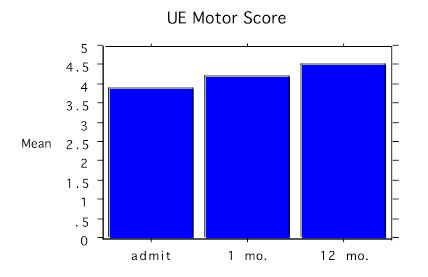
Outcome Motor strength on a 0–5 ASIA motor scale increased in the lower limbs from 3.6 standard deviation (SD)± 0.5 to 4.2 SD ± 0.4 and in the upper limbs from 3.9 SD ± 0.3 to 4.5 SD ± 0.5 on the ASIA motor score and was consistently maintained over 12 months (Figures 1 & 2). Almost all the motor changes were confined to the lower extremities except for dorsiflexion, which did not change.
The patient reported considerable increases in walking endurance, decreased falls and no longer uses a cane on level surfaces for ambulation. There was a mild decrease in the perception of pain as measured by the Visual Analog scale from 4 to 3 in the feet and from 7 to 5 in the hands with no change in pain medications. Much of this change in pain was a reduction in muscle cramping rather than in the burning dysaesthesia. The patient reported that he was able to now ambulate on level surfaces for more than one block without his single point cane and was able to walk on uneven surfaces with his single point cane. Subjectively he reported feeling the best he had in the last 10 years.
Figure 1. Bar chart with the change in lower extremity (LE) average motor score before treatment (admit), and at 1 month (mo.) and 12 months
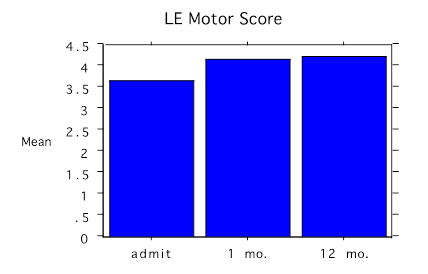
Figure 2. Bar chart with the change in upper extremity (UE) average motor score before treatment (admit), and at 1 month (mo.) and 12 months
Laboratory testing failed to reveal any change in the hemoglobin, hematocrit, red blood cell count, white blood cell count with differential and platelet count, sodium, potassium, chloride, bicarbonate, glucose, urea nitrogen (BUN), creatinine, total protein, albumin, aspartate aminotransferase (SGOT/AST), alanine aminotransferase (SGPT/ALT), alkaline phosphatase, uric acid, phosphorous (PO4), albumin, calcium, total bilirubin and lactate dehydrogenase (LDH). There was also no change in the electrocardiogram during the study.
Discussion
This case report indicates 4-aminopyridine is effective in improving the motor function of a patient with CMT. While this was an open label trial the patient clearly felt better with the medication and has continued to take it for over one year now. He reported that it had been the best year in the last ten. It is unfortunate that we did not perform before and after functional tests such as a 6-minute walking test or a Get up and Go test, as it may have measured endurance as well as motor strength.
It is felt that the mechanism of 4-AP may in part be due to the fact that it allows for a longer period of sustained voltage thereby supplying enough electrons to conduct via saltatory conduction beyond the point of demyelination to continue the action potential distally. As such 4-AP may usefulness in many demyelinating diseases such as subgroups of CMT, as well as multiple sclerosis or GBS.
4-AP is available on the FDA compounding list from a reputable compounding pharmacy. Adverse effects associated with oral administration of 4-AP have included mild dizziness, light-headedness, paresthesia/dysesthesia, nausea and mild agitation.4,5,13,14 Doses above 30 mg. per day have induced confusional states (disorientation, agitation, anxiety), respiratory distress (dyspnea, hyperventilation), locomotor and balance problems and epileptiform seizures.15-18 The seizures appear to be most prevalent in those who have multiple sclerosis.4,5,14 Cardiac abnormalities have been reported only with extremely high doses beyond what is now recommended for treatment.7 There is usually no disturbance in autonomic function with CMT.2
4-AP is available in a compounded form in the United States. It is similar to the drug dalfampridine utilized in multiple sclerosis. It readily crosses the blood nerve barrier as well as the blood brain barrier and appears to reach higher interstitial concentrations than dalfampridine due its ability to reach higher serum levels pushing it across.19 It has been suggested for use in the treatment of peripheral neuropathies, predominately those involving demyelination. It has also been utilized in multiple sclerosis20 and generally is available at 1/10 the cost.
It is not expected that 4-AP will slow the progression of the disease. Nor did it have a significant effect on the patient’s pain perception. He did not experience a reduction in the “cramping sensations” he experienced after walking. This is a predominant symptom of the disease.2,3 Further research requires delineating time length of the biologic effects of 4-AP on motor function, which may be three weeks long.12
Clearly more research including prospective trials is required in CMT and its various subtypes with the use of 4-AP. It has the potential to improve motor function in these patients and thereby reducing disability. This case report indicates that this work should go forward.
References
- Pareyson D. Charcot-Marie-Tooth disease and related neuropathies: Molecular basis for distinction and diagnosis. Muscle Nerve 1999;22(11): 1498–1509.
- Adams RD, Victor M. Principles of Neurology. 5th ed. New York, NY: McGraw-Hill; 1993.
- Dyck PJ, Chance P, Lebo R, et al. Hereditary motor and sensory neuropathies. Peripheral Neuropathy. 3rd ed. Dyck PJ, Thomas PD, et al, Eds. 1993: 1094–1135.
- Davis FA, Stefoske D, Rush J. Orally administered 4-aminopyridine improves clinical signs in multiple sclerosis. Ann Neurol 1990;27(2): 186–192.
- Jones RE, Heron JR, Foster DH, et al. Effects of 4-aminopyridine in subjects with multiple sclerosis. J Neurol Sci 1983; 60: 353–362.
- Schwid SR, Petrie MD, McDermott MP, et al. Quantitative assessment of sustained-release 4-aminopyridine for symptomatic treatment of multiple sclerosis. Neurology 1997;48(4): 817–821.
- Bever CT Jr. The current status of studies of aminopyridines in patients with multiple sclerosis. Ann Neurol 1994;36(Suppl): S118–S121.
- Goodman AD, Brown,TR, Edwards KR, et al. A phase 3 trial of extended release oral dalfampridine in multiple sclerosis. Ann Neurol 2010;68(4): 494–502.
- Meythaler JM, Brunner RC. Improvement of Motor, Sensory, and Functional Status of Post-Guillain-Barre Syndrome with the Use of 4-Aminopyridine. Int J Phys Med Rehabil 2014;2: 202.
- Hansebout RR, Blight AR, Fawcett S, et al. 4-aminopyridine in chronic spinal cord injury: a controlled, double-blind, crossover study in eight patients. J Neurotrauma 1993;10(1): 1–18.
- Waxman SG. Aminopryridines and the treatment of spinal cord injury. J Neurotrauma 1993;10: 19–24.
- Segal J, Pathak MS, Hernandez JP, et al. Safety and efficacy of 4-aminopyridine in humans with spinal cord injury: a long-term, controlled trial. Pharmacotherapy 1999;19(6): 713–723.
- Sanders DB, Kim YI, Howard JF, et al. Eaton-Lambert syndrome: A clinical electrophysiological study of a subject treated with 4-aminopyridine. J Neurol Neurosurg Psychiatr 1980;43: 987–995.
- Stefoski D, Davis FA, Fitzsimmons WE, et al. 4-aminopyridine in multiple sclerosis: Prolonged administration. Neurology 1991;41(9): 1344–1348.
- Lundh H, Nilsson O, Rosin I. Effects of 4-aminopyridine in myesthenia gravis. J Neurol Neurosurg Psychiatr 1979;42: 171–175.
- Ball AP, Hopkinson RB, Fareel ID, et al. Human botulism caused by clostridium botulinum type e - The Birmingham Outbreak. Quart J Med 1979;48(191): 473–491.
- Murray NMF, Newsom-Davis J. Treatment with 4-aminopyridine in disorders of neuromuscular transmission. Neurology 1981;31(3): 265–271.
- Spyker DA, Lynch S, Shabanowitz J, et al. Poisoning with 4-aminopyridine: Report of three cases. Clin Toxicol 1980;16(4): 487–497.
- Meythaler JM. Treatment of a neuropathy with rapid release aminopyridine. United States Patent and Trademark Office. US: 20110003868; 1/6/2011.
- Polman CH, Bertelsmann FW, van Loenen AC, et al. 4-aminopyridine in the treatment of patients with multiple sclerosis. Long-term efficacy and safety. Arch Neurol 1994;51(3): 292–296.



