Home / Journal / Cancer Studies
The Role of Metastasectomy in Women with Solitary Metastatic Breast Cancer Recurrence
metastasectomy distant recurrence survival
Patrick Mun Yew Chan, Sherwin Kuah, Mui Heng Goh, Sarah Qinghui Lu, Juliana Jia Chuan Chen and Ern Yu Tan
DOI: https://doi.org/10.31532/CancerStud.1.1.005 12 Jan 2018
Abstract
Background: Complete resection of solitary metastasis can render a woman disease-free, raising the possibility that curative treatment is still possible even in metastatic breast cancer. However, there is no conclusive data in support of this and the actual value of metastasectomy is uncertain since it is often performed only in women expected to have a more favorable outcome. Aims: In this present study, we evaluated 13 women who underwent metastasectomy after relapsing with solitary metastasis. The endpoints of disease progression after metastasectomy and overall survival were specifically examined. Results: All 13 women had previously undergone curative treatment for breast cancer. Ten women were known to have locally advanced Stage III disease. The distant recurrence was diagnosed after new symptoms developed in 12 women; the remaining woman was found with asymptomatic mediastinal nodal recurrence on positron emission tomography-computed tomography. Complete resection of all gross tumour was done in all instances and 9 women received further systemic treatment after surgery. All 5 women with brain metastasis received whole brain radiation after surgery. New metastatic disease developed in 8 women and was adjacent to the previous resection bed in 3 women. No further surgery was done in these 8 women. Median overall survival for the 13 women was 74.70 months (ranging from 32.07 to 336.80 months) and the women survived a median of 30.57 months (ranging from 16.60 to 80.40 months) after metastasectomy. There was a non-significant trend towards better overall survival in these 13 women compared to others who had received only systemic treatments (P = 0.398). Conclusions: Reasonable survival was observed in women who had undergone metastasectomy. Complete resection of metastatic tumour can be considered in selected women with limited secondary metastases from breast cancer.
Keywords
Metastasectomy, distant recurrence, survival
Background
Despite various advances in treatment modalities, distant metastasis continues to be the leading cause of mortality in women with breast cancer. Systemic relapse occurs in up to 25% of women with early stage breast cancer and the incidence is even higher in those with Stage III disease or triple negative cancers.1 Disease is often disseminated at the time of systemic failure and treatment is primarily palliative with chemotherapy, targeted therapy and/or hormonal therapy. Surgery is generally reserved for the relief of symptoms and for the prevention of debilitating complications, such as spinal cord compression and hip fractures.2 Median survival is 2 to 4 years, but can vary widely and women with oligometastatic disease and those with disease limited to the bones often have a more favourable outcome.3 Rarely, the metastatic disease is limited to a single organ and is potentially operable. While there is no definite evidence that metastasectomy improves survival, complete resection to achieve a disease-free state is at times considered especially in younger women and those with good performance status. Retrospective studies have reported good survival following complete resection of solitary metastatic deposits, however the benefit of surgery is debatable as these women were selected based on factors predictive of a more favourable prognosis.2,4,5 No prospective data is available and the low frequency of such cases makes it unlikely that large prospective or randomised trials will be possible even in the future. Furthermore, most reports evaluated women presenting with de novo metastatic disease, rather than with distant recurrence, and the two may not be comparable since treatment resistance is more likely to be encountered in recurrent tumours.6
Isolated distant recurrence is an uncommon event, particularly in the current setting where highly sensitive imaging modalities, such as positron emission tomography-computed tomography (PET/CT), are used for staging. In the absence of disease elsewhere, complete resection of the isolated metastasis can render the patient disease-free and may confer a survival benefit. With increasing expertise in minimally invasive surgery and improvements in peri-operative management, even major resections can be done with acceptable risks and morbidity and consequently, resection of asymptomatic metastases may be justifiable. Given the paucity of data on the benefit of metastasectomy, we have described our experience in a series of women who underwent complete resection of isolated breast cancer metastasis. In this present study, we examined the outcome after metastasectomy, focusing specifically on disease progression after surgery and on overall survival.
Materials and Methods
A total of 322 women who developed systemic relapse (distant recurrence) from between 1st January 2005 to 31st December 2016 were identified from the TTSH Breast Cancer Prospective Database (TTSH/2017-00011). Ethics approval for this study was obtained from the National Healthcare Group Domain Specific Review Board (DSRB/2010/00031). From amongst these, we identified 13 women who were considered disease-free after metastasectomy. All had solitary or limited distant metastasis to a single organ and had undergone complete resection of all gross tumour. Another 216 women received systemic treatment but no surgery and a further 93 women did not receive any treatment (33 were deemed medically unfit, 35 died prior to the initiation of treatment, 20 refused further treatment and the remaining 5 women sought a second opinion outside of our institute).
All 13 women were previously diagnosed with breast cancer and had been treated with curative intent. Surgery consisted of either wide local excision (WLE) or mastectomy and other adjuvant treatments were recommended in accordance with the existing NCCN Guidelines. Anthracycline-based chemotherapy regimens were the most frequently used. One patient diagnosed before 2006 received cyclophosphamide / methotrexate / fluorouracil (CMF) and another patient received fluorouracil / doxorubicin / cyclophosphamide (FAC). From 2006 onwards, doxorubicin / cyclophosphamide (AC) followed by paclitaxel was the preferred regimen, and was given as a dose-dense regimen at the discretion of the oncologist. The non-anthracycline based docetaxel / cyclophosphamide (TC) was also used, particularly when there were concerns about cardiotoxicity. Also, from 2006 onwards, HER2-testing became routine and trastuzumab (17 cycles over 12 months) was offered to women with HER2-overexpressing tumours. Women with ER-positive tumours were recommended hormonal therapy. Tamoxifen was the agent in use prior to 2006, thereafter, aromatase inhibitors (anastrozole, letrozole) were increasingly offered to post-menopausal women. A 5-year regimen was the standard of care during the study period. Whole breast radiation, total of 50Gy administered in 25 fractions and a 10Gy boost to the tumor bed, was standard after WLE. Post-mastectomy radiation was recommended in instances where the tumor was more than 5cm in size, when there was underlying muscle involvement and when there was nodal involvement (discussed on a case-by-case basis in post-menopausal women with less than 4 involved nodes). Chest wall irradiation of 50Gy in 25 fractions was given, with no additional boost. Nodal irradiation, given in node-positive disease, comprised of 3 fields to cover the axillary nodal basin and supraclavicular fossa, or 4 fields to also include the posterior axilla. The internal mammary nodes were not routinely included unless for medially located tumors and when there was evidence of internal mammary involvement.
Surveillance consisted of regular clinical examinations and annual mammography, with complementary breast ultrasound done in selected instances. The women were reviewed at our unit every 4 to 6 months for the first 5 years and annually after 5 years, but they were advised to return for an early consult whenever any new symptoms arose. In our local practice, cancer surveillance is carried out at the tertiary centre were the women had received treatment. Serial measurements of tumour markers such as CA15-3 and CEA, liver function and repeat CT scan evaluations were not routine and were done at the discretion of the oncologist. Consequently, systemic relapse was often diagnosed only when symptoms developed or when re-staging scans were done after a local recurrence was detected. Computed tomography and bone scan were standard first-line imaging modalities, while magnetic resonance imaging (MRI) and positron emission tomography (PET)/CT were used selectively. The diagnosis of distant recurrence was made when lesions compatible with metastasis were detected on imaging scans; histological confirmation was not mandatory unless the imaging findings were indeterminate. Treatment following the diagnosis of distant recurrence was discussed at the multidisciplinary board meetings. Systemic treatment (chemotherapy, trastuzumab, hormonal therapy, either as monotherapy or in combination) was the standard recommendation, although metastasectomy was considered in selected cases where there was limited and resectable systemic disease in younger women with no or minimal co-morbidities and good performance status. Decision for metastasectomy was made after discussion with the patient and family and no standard criteria was used. The median follow-up period for this study was 108.10 months (ranging from 33.07 to 337.80 months).
Kaplan-Meier survival analysis was used to evaluate differences in overall survival between women who had undergone metastasectomy and those who had received systemic treatments alone and was performed with GraphPad Prism version 6 (GraphPad software Inc., San Diego, CA, USA). Time to progression was calculated from the time of metastasectomy to the detection of new metastasis. Overall survival was calculated from the time of treatment initiation (of the initial tumour) to the time of death or the date of last follow-up. A P value of less than 0.05 was considered statistically significant.
Results
We identified 13 women with invasive breast cancer who subsequently developed solitary distant recurrence. One patient had node-negative Stage I (T1cN0) disease (Patient 1), 11 had node-positive disease (2 Stage IIB, 9 Stage III) and no information was available for the last patient who was previously treated outside our unit. Details of the 13 women, initial cancer stage and characteristics are listed in Table 1. All 13 women were initially treated with curative intent. Details of treatments received are listed in Table 2. Treatment had been initiated within 1 month of diagnosis (median of 0.87 months) for all women except Patient 2, who started neoadjuvant chemotherapy 5.97 months after diagnosis; she had sought alternative treatment initially. All 13 women had undergone surgery (mastectomy in all except Patient 2, who underwent WLE) and all had received chemotherapy; 8 as neoadjuvant treatment. Doxorubicin/cyclophosphamide (AC) followed by paclitaxel was the most frequently used regimen, whether in the neoadjuvant or adjuvant setting. Five of 6 women with HER2-overexpressing tumours received trastuzumab; Patient 1 had declined trastuzumab. Of the 6 women with ER-positive tumours, 5 received hormonal therapy and Patient 9 relapsed before it was started. Eleven women received post-operative radiation. In all instances, the ipsilateral axilla and supraclavicular fossa were included (3 fields); the posterior axilla was also included in 4 women (4 fields).
Table 1. Clinicopathological details of the 13 patients
Parameter |
Number |
Median age (years) |
50 (36 – 67) |
Ethnicity Chinese Malay Indian Others |
11 1 0 1 |
Disease Stage I IIB
IIIA |
1 2 3 2 4 1 |
Extent of nodal involvement N0 N1
N2 |
1 6 1 4 1 |
Median number of nodes involved |
5 (1 – 42) |
Tumour Histology Invasive ductal carcinoma Invasive lobular carcinoma Unknown |
11 1 1 |
Median tumour grade |
2 (1 – 3) |
ER status Positive Negative Unknown |
6 6 1 |
PR status Positive Negative Unknown |
3 9 1 |
HER2 status Positive Negative Unknown |
6 4 3 |
Table 2. Details of the 13 women who underwent metastasectomy
Patient |
Stage |
Chemotherapy* / Trastuzumab (status) |
Hormonal therapy |
Radiation (status) |
Site of distant recurrence (TTDR) |
Metastectomy done |
Treatment after metastectomy |
Progression after metastasectomy (TTP) |
Treatment after progression |
Overall survival (months) |
1 |
I |
ACx4;Tx12 |
Tamoxifen |
No |
Sternum(14.10) |
Curettage with bone grafting |
GemcitabineTrastuzumab |
Sternum(4.63) |
RTGemcitabineTrastuzumab |
52.97 |
2 |
IIB |
Neoadjuvant ACx4;Tx12 |
Noa |
50+10Gy;3 fields |
Mediastinal node(20.50) |
VATS metastatectomy |
Cisplatin/Gemcitabine |
No |
|
37.40 |
3 |
IIB |
NeoadjuvantACx4;Ixabepilone x4 |
Letrozole |
50Gy; 3 fields |
Left temporo-parietal lobe (25.67) |
Craniotomy and excision |
Whole brain RT Letrozole |
No |
|
56.63 |
4 |
IIIA |
Neoadjuvant ACx4; Tx12 Trastuzumab |
Noa |
50Gy; 3 fields |
Lung, left upper lobe (29.00) |
VATS metastatectomy |
TrastuzumabAnastrozole |
Mediastinal and hilar nodes(33.67) |
LapatinibAromasin |
110.70 |
5 |
IIIA |
Neoadjuvant ACx4; Tx4 |
Tamoxifen |
50+10Gy; 4 fields |
Left Hip(30.43) |
Tumour resection and reconstruction of left pelvis |
Letrozole |
Lungs(28.67) |
Xeloda |
74.70 |
6 |
IIIA |
ACx4; Tx4 Trastuzumab |
Noa |
50Gy;3 fields |
Right temporal lobe(22.97) |
Craniotomy and excision of tumour |
Whole brain RT |
Cervical nodes(5.63) |
GemcitabineTrastuzumab |
89.10 |
7 |
IIIB |
Neoadjuvant CMFx6 |
Tamoxifen† |
50Gy;3 fields |
Right fronto-temoporal lobe(65.80) |
Craniotomy and excision of tumour |
Whole brain RTChemotherapydLetrozole |
Brain(20.00) |
No |
96.30 |
8 |
IIIB |
Neoadjuvant ACx4; Tx12Trastuzumab** |
Noa |
50Gy; 3 fields |
Right frontal lobe(15.63) |
Awake craniotomy and excision of tumour |
Whole brain RT |
Bone(4.47) |
RT |
40.63 |
9 |
IIIC |
Neoadjuvant TCx4 |
Nob |
14Gy; 4 fields** |
Contralateral axilla node (7.60) |
Left axillary clearance |
Axillary RTAnastrozole |
Mediastinal nodes(24.27) |
GemcitabineAromasin |
67.63 |
10 |
IIIC |
Neoadjuvant FACx6 |
Noa |
50Gy; 4 fields |
Left cervical node (117.93) |
Radical neck dissection |
Tx12Trastuzumab |
No |
|
173.93 |
11 |
IIIC |
ACx4; Tx12 Trastuzumab |
Tamoxifen† |
50Gy;3 fields |
Left branchial plexus (78.03) |
Clavicular oestomy, brachial plexus exploration, neurolysis and excision of metastatic mass |
XelodaTrastuzumabTamoxifen |
Brachial plexus(3.43) |
VinorelbineTrastuzumabTamoxifen |
97.50 |
12 |
IIIC |
TCx1** |
Noa |
50Gy; 4 fields |
Left parietal lobe(3.80) |
Craniotomy and excision of brain tumour |
Whole brain RT |
No |
|
32.07 |
13 |
Unknown |
Yes, regimen unknown |
Noc |
No |
Lung, right lower lobe (303.17) |
Robotic assisted lower lobectomy and lymph node dissection |
No |
No |
|
336.80 |
*Chemotherapy regimens. ACx4; Tx12: doxorubicin / cyclophosphamide (AC) 4 cycles followed by weekly paclitaxel 12 cycles; ACx4; Tx4: dose-dense AC 4 cycles followed by paclitaxel every 2 weeks 4 cycles; TCx4: docetaxel / cyclophosphamide 4 cycles; CMFx6: cyclophosphamide / methotrexate / fluorouracil 6 cycles; FACx6: fluorouracil / doxorubicin / cyclophosphamide 6 cycles. **denotes premature discontinuation of treatment regimen. †completed 5 years of hormonal therapy. a ER-negative tumour; b Relapse prior to starting hormonal therapy; c Presumably ER-negative (no details available); d Treated outside our unit, regimen details not available. RT: radiation. TTDR: time to distant recurrence (months); TTP: time from metastasectomy to progression (months).
Overall, 7 women had completed the recommended chemotherapy, trastuzumab and radiation treatments. 1 woman (Patient 12) discontinued docetaxel/cyclophosphamide (TC) treatment after the first cycle because of intolerable side effects and 3 women did not complete 1 year of trastuzumab. Radiation was prematurely discontinued in Patient 9 after contralateral axillary nodal metastases were found and hormonal therapy was also not started until after axillary clearance. Two women with ER-positive disease relapsed after completing 5 years of tamoxifen, while the other 3 women had relapsed still on hormonal therapy.
The 13 women developed distant recurrence a median of 22.10 months (ranging from 2.23 to 303.17 months) after the initiation of treatment (either neoadjuvant chemotherapy or surgery). Systemic disease involved the brain, lungs, bone, and lymph nodes (cervical, mediastinal and contralateral axilla) (Table 2). Patient 9 had developed chest wall recurrence 6 months prior to the distant recurrence and re-staging scans done at the time of the local recurrence had shown no metastasis. Other than Patient 2, in whom asymptomatic mediastinal nodal recurrence was detected on PET/CT imaging and Patient 9, in whom the contralateral axillary node was detected by the clinician, all the other women had presented with symptoms. The 5 women (Patients 3, 6, 7, 8 and 12) with brain metastasis were diagnosed after developing symptoms; 2 complained of headache, 2 presented with slurred speech and 1 had seizures. Patient 4, diagnosed with lung metastasis, had complained of a chronic cough and Patient 13 had complained of weight loss. Patient 10 presented with a self-detected palpable cervical node and Patient 9 was found with a palpable axillary node while undergoing post-mastectomy radiation. Patient 11 was found with supraclavicular and axillary nodal recurrence encasing the brachial plexus after she presented with intractable left upper arm pain and numbness. Patient 1 presented with central chest pain and Patient 5 presented with left hip pain.
Surgery was performed as detailed in Table 2. In all instances, the gross tumour was completely resected and was confirmed histologically as a breast cancer recurrence, being morphologically similar to the primary tumour. Receptor status was repeated on the recurrent tumour in 6 cases and was discordant in 3. All 13 women were considered disease-free after metastasectomy. Nine women received further systemic treatment after metastasectomy; 6 received chemotherapy or targeted therapy and another 3 received hormonal therapy alone. All 5 women with brain metastasis received whole brain radiation following surgery.
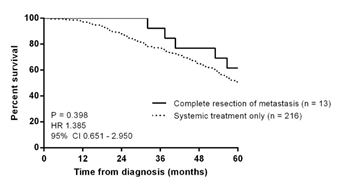
Eight women developed new metastasis after surgery, after a median interval of 12.82 months (ranging from 3.43 to 33.67 months). In 3 patients (Patients 1, 7, and 11), the tumor recurred adjacent to the previous resection bed. No further resection was attempted and all 8 women were treated with further systemic treatment. Two patients (Patient 1 and Patient 8) received radiation to the bone for pain control. Death occurred in 7 women, 6 of whom had developed new disease after metastasectomy. Median interval from progression to death was 26.48 months (ranging from 8.67 to 59.33 months). Median overall survival for the group was 74.70 months (ranging from 32.07 to 336.80 months) and the women survived a median of 30.57 months (ranging from 16.60 to 80.40 months) after metastasectomy. Comparison was made with 216 women diagnosed with distant recurrence during the same period but who had received systemic therapy alone (no resection of metastasis). Treatment details for these 216 women are as follows: 71 received hormonal monotherapy, 51 received chemotherapy, 24 received chemotherapy and trastuzumab, 21 received chemotherapy, trastuzumab and hormonal therapy, 47 received chemotherapy and hormonal therapy and 2 received trastuzumab and hormonal therapy. There was a trend towards better 5-year overall survival in the 13 women who had undergone metastasectomy, but this was not statistically significant (P = 0.398) (Figure 1).
Figure 1. Kaplan-Meier curves of 5-year overall survival in women with distant recurrence, stratified by metastasectomy versus systemic treatment only (n = 229)
Discussion
In this present study, we described a series of 13 women who relapsed with operable metastasis in a single organ. There was a non-significant trend towards better overall survival compared to women who had received only systemic treatment. New systemic disease developed in more than half the women after a median of 12.8 months and these women survived for a further 10.5 to 48.0 months. Overall survival was 74.7 months, with women surviving a median of 30.6 months after metastasectomy.
Resection of metastasis achieves a disease-free state, but it is not known whether this prolongs overall survival. In the primary setting, complete resection of the breast tumour allows systemic therapies to more effectively control and eliminate micrometastasis and this is thought to also apply to solitary metastatic disease. Conflicting results have been reported when the primary breast tumour is resected, but leaving behind metastatic lesions, and no clear benefit in terms of disease control or overall survival has been demonstrated.7-9 There is little data on the outcome of resecting both the primary and metastatic tumours. In colorectal cancer, resection of secondary liver metastases is of proven survival benefit and could support the rationale for aggressive local treatment of operable metastases.10 However, spread to the liver occurs via the portal vein that drains the colon and consequently, liver metastasis can be considered more of a regional spread rather than systemic dissemination, as in the case of secondary metastasis from the breast. Unlike with colorectal cancer, there is no dominant site of metastasis and metastasis to the liver and brain are often late events in breast cancer. As such, resection of secondary metastases from breast cancer may not produce the same survival gain as seen in colorectal cancer. Despite this, a survival benefit was still demonstrated after curative resection of extra-hepatic lung metastasis from colorectal cancer, suggesting that metastasectomy might still have a role in disseminated disease when it is limited.11
Reports of metastasectomy have been in the setting of de novo metastatic disease, rather than distant recurrence. Resections of solitary metastases involving the lungs, liver and brain are most frequently reported. Five-year survival rates of 27% to 80% have been reported following complete anatomical resection of solitary or limited lung metastases.4,12,13 Median survival following complete resection of liver metastases was similarly encouraging, with 5-year overall survival rates of up to 60% being reported.5,14,15 However, these data were derived from a highly selected cohort of women, who had oligometastatic disease affecting a single organ, were young, had good performance status, and often had ER-responsive tumors and a long disease-free interval prior to relapse.16–18 These factors in themselves are associated with a more favorable prognosis and there is a question of whether metastasectomy actually confers any further survival advantage.
Unlike with lung and liver metastasis, which are often asymptomatic when operable, brain metastases are often diagnosed only when symptoms develop, as was the case in all 5 patients in our study. Tumor resection is therefore necessary to relieve or control new onset neurological symptoms. Whole brain radiation after surgery confers added control and improves survival.19 All 5 patients in our study received whole brain radiation after complete resection of the brain metastasis, but in spite of this, tumor recurrence occurred adjacent to the previous resection site in one patient. This patient had also received chemotherapy and hormonal therapy following craniotomy. This case highlights the difficulties encountered in treating brain metastasis, where the adequacy of resection is limited by potential morbidity and drug penetration of the blood-brain barrier is often suboptimal.
Surgery for bone metastasis is primarily undertaken to relieve spinal cord compression, severe intractable pain and to prevent an impending fracture at a weight-bearing site. Solitary bone metastasis is uncommon and the experience with resection of asymptomatic bone metastasis is very limited. Some studies have suggested a role for resection of solitary sternal metastasis,20,21 but the associated morbidity and cosmetic deformity can be significant. A less extensive procedure, such as curettage, may not be adequate as suggested by the one patient in our study who had tumor recurring adjacent to the surgical bed only 5 months after the first surgery. Complete resection of the bone containing the metastasis would appear superior. No recurrence developed in the one patient who underwent resection of the left pelvis. This patient later relapsed in the lungs about 29 months later, but survived for another 46 months after that. There is little of data on the resection of nodal metastases. We achieved good outcomes with the resection of axillary, mediastinal and cervical nodal metastases and two patients did not progress further. On the other hand, complete resection of axillary and/or supraclavicular nodal metastases may be difficult when there is encasement of the brachial plexus and the short interval from surgery to tumour recurrence, as seen in our patient, would perhaps suggest that radiation may be more appropriate for symptomatic control.
Conclusions
In our present study, we observed reasonably good outcomes after metastasectomy. More than half the women later developed new metastasis, but median survival after progression was 27 months. It will likely remain difficult to define the benefit of metastasectomy and it seems a reasonable consideration in women expected to have long-term survival and when complete resection carries minimal morbidity. Risk stratification has primarily been explored in de novo metastatic disease and it remains to be determined whether these factors also identify a subgroup of women who would benefit from complete resection of secondary breast metastases.
Acknowledgments
None.
Funding Information
None.
Conflicts of Interest
All authors declare no conflicts of interest.
References
- Rugo HS. The importance of distant metastases in hormone-sensitive breast cancer. Breast 2008;17 Suppl 1: S3–S8.
- Pagani O, Senkus E, Wood W, et al. International guidelines for management of metastatic breast cancer: can metastatic breast cancer be cured? J Natl Cancer Inst 2010;102(7): 456–463.
- Leung AM, Vu HN, Nguyen KA, et al. Effects of surgical excision on survival of patients with stage IV breast cancer. J Surg Res 2010;161(1): 83–88.
- Yoshimoto M, Tada K, Nishimura S, et al. Favourable long-term results after surgical removal of lung metastases of breast cancer. Breast Cancer Res Treat 2008;110(3): 485–491.
- Chua TC, Saxena A, Liauw W, Chu F, Morris DL. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer 2011;47(15): 2282–2290.
- Tanei T, Morimoto K, Shimazu K, et al. Association of breast cancer stem cells identified by aldehyde dehydrogenase 1 expression with resistance to sequential Paclitaxel and epirubicin-based chemotherapy for breast cancers. Clin Cancer Res 2009;15(12): 4234–4241.
- Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. Lancet Oncol 2015;16(13): 1380–1388.
- Soran O, Ozmen V, Ozbas S, et al. A randomized controlled trial evaluating resection of the primary breast tumor in women presenting with de novo stage IV breast cancer. J Clin Oncol 2016;34S: ASCO #1005.
- King TL, Layman J; Gonen M, et al. A prospective analysis of surgery and survial in stage IV breast cancer (TBCRC 013). J Clin Oncol 2016;34S: ASCO #1006.
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 2007;25(29): 4575–4580.
- Wiegering A, Riegel J, Wagner J, et al. The impact of pulmonary metastasectomy in patients with previously resected colorectal cancer liver metastases. PLoS One 2017;12(3): e0173933.
- Friedel G, Pastorino U, Ginsberg RJ, et al, International Registry of Lung Metastases LE. Results of lung metastasectomy from breast cancer: prognostic criteria on the basis of 467 cases of the International Registry of Lung Metastases. Eur J Cardiothorac Surg 2002;22(3): 335–344.
- Meimarakis G, Ruttinger D, Stemmler J, et al. Prolonged overall survival after pulmonary metastasectomy in patients with breast cancer. Ann Thorac Surg 2013;95(4): 1170–1180.
- Yoshimoto M, Tada T, Saito M, et al. Surgical treatment of hepatic metastases from breast cancer. Breast Cancer Res Treat 2000;59(2): 177–184.
- Veltri A, Gazzera C, Barrera M, et al. Radiofrequency thermal ablation (RFA) of hepatic metastases (METS) from breast cancer (BC): an adjunctive tool in the multimodal treatment of advanced disease. Radiol Med 2014;119(5): 327–333.
- Hortobagyi GN. Can we cure limited metastatic breast cancer? J Clin Oncol 2002;20(3): 620–623.
- Ly BH, Nguyen NP, Vinh-Hung V, et al. Loco-regional treatment in metastatic breast cancer patients: is there a survival benefit? Breast Cancer Res Treat 2010;119(3): 537–545.
- Miao H, Hartman M, Bhoo-Pathy N, et al, Verkooijen HM. Predicting survival of de novo metastatic breast cancer in Asian women: systematic review and validation study. PLoS One 2014;9(4): e93755.
- Wronski M, Arbit E, McCormick B. Surgical treatment of 70 patients with brain metastases from breast carcinoma. Cancer 1997;80(9): 1746–1754.
- Noguchi S, Miyauchi K, Nishizawa Y, et al. Results of surgical treatment for sternal metastasis of breast cancer. Cancer 1988;62(7): 1397–1401.
- Noble J, Sirohi B, Ashley S, et al. Sternal/para-sternal resection for parasternal local recurrence in breast cancer. Breast 2010;19(5): 350–354.



