Home / Journal / Physical Medicine and Rehabilitation
Feasibility of a Group-based Telerehabilitation Intervention for post-COVID Condition Management
COVID-19 Post-COVID Condition Long-COVID Rehabilitation Telerehabilitation Exercise Therapy Physical Therapy
Meredith F. King, Anthony Byrne, Linda Denehy, Petra L. Graham, Benjamin Douglas, Priscilla de Toni, Angelo R. Santos, Emily Kitson, Alison Pang, Elena M. Bartos, Marshall Plit, Sean F. Mungovan
DOI: 10.31532/PhysMedRehabil.2.1.005 25 Sep 2023
Abstract
Background and objective: The prevalence and chronic nature of post-COVID condition (PCC) burden requires a better understanding of effective rehabilitation interventions in large scale clinical trials. Telerehabilitation exercise-based interventions are effective in other chronic disease groups. The aim of this study was to assess the feasibility of a physiotherapist-supervised group telerehabilitation exercise intervention for PCC management. Methods: Individuals presenting with PCC symptoms, defined as persistent dyspnoea, fatigue or chest pain, beyond 4 months (120 days) post COVID-19 diagnosis and had not undertaken exercise training three months prior to randomisation into the trial were included. Participants were randomised to receive either i) a twice-weekly 45 min physiotherapist-supervised group telerehabilitation exercise sessions via the Zoom teleconferencing platform for ten weeks or iii) continue with usual care. Feasibility outcomes included recruitment rate, adherence, completion rate, adverse events and technological issues. Exploratory clinical outcomes including exercise capacity, fatigue and health-related quality of life were assessed. Results: 21 participants with mean age 53 ± 14 yrs were recruited 365 ± 67 days after a diagnosis of COVID-19. The recruitment rate was 39% of possible participants. The telerehabilitation participants completed 18 ± 2 sessions, with 100% completing 16 sessions or more. There were no adverse events and two technological problems reported for the intervention group. Nine (82%) of the intervention group completed the follow-up assessments. Conclusion: Supervised group telerehabilitation is feasible and safe for PCC management. Future trials are required to investigate the efficacy and generalizability of the supervised group-based telerehabilitation exercise intervention for PCC management.
Keywords
COVID-19; Post-COVID Condition; Long-COVID; Rehabilitation; Telerehabilitation; Exercise Therapy; Physical Therapy
Introduction
The COVID-19 pandemic has resulted in an unprecedented global health crisis, impacting health care systems with over 769 million people infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and close to 7 million related deaths reported globally prior to August 16 2023.1 COVID-19 disease, caused by SARS-CoV-2 can result in multiple organ dysfunction and cause a wide spectrum of acute symptoms at presentation.2 Early research efforts in the COVID-19 pandemic were focussed on managing the acute illness and reducing mortality. However, chronic morbidity, persisting long after the acute infection period and unrelated to the severity of the index illness is now an important health concern.3 Persistent symptoms of residual COVID-19 include shortness of breath, persistent fatigue, “brain fog”, muscular weakness, chest pain, cognitive impairment and joint pain, resulting in reduced physiological, mental and functional capacity as well as health related quality of life (HRQoL).4,5 Post-COVID condition (PCC) or Long-COVID are terms used to describe the persistent symptoms extending three months beyond the acute period of infection. The incidence of PCC remains contested with studies reporting a range of 10-20% among non-hospitalised patients6,7 and 51-76% of patients reporting at least one symptom at four months following hospitalisation8,9 Regardless of the initial COVID-19 disease severity,10 PCC presentations appear similar to that of post-intensive care syndrome including impairments in physical, emotional and cognitive health and wellbeing.11
Given the incidence of SARS-CoV-2 globally, there is now a significant number of individuals who are at risk of developing PCC with implications for rehabilitation service delivery. There is an urgent need to better understand the feasibility and safety of interventions to manage PCC presentations. Exercise-based rehabilitation programs are highly effective to reduce respiratory symptoms and improve physiological functional capacity12–14 and such programs can be delivered effectively in outpatient/community settings and via telehealth platforms.15,16 For COVID-19, international guidelines recommend access to rehabilitation services for individuals with persistent COVID-19 symptoms17 and there is a need for studies investigating the effects of different rehabilitation programs.18
This randomised pilot trial aimed to evaluate the feasibility of a physiotherapist-supervised group telerehabilitation exercise intervention for individuals with PCC. A secondary aim was to collect descriptive statistics on clinical outcomes that may be relevant for planning future trials.
Methods
Trial Design
This was a prospective, single centre, randomised pilot feasibility trial that was undertaken between March 2021 and August 2021. The reporting of this pilot feasibility trial followed the Consolidated Standards of Reporting Trials for pilot and feasibility studies (CONSORT)19 and Template for Intervention Description and Replication (TIDieR) guidelines.20 The pilot feasibility trial was approved by the Sydney Local Health District Human Research Ethics Committee (X20-0545 & 2020/ETH03228) and was registered prospectively with the Australian and New Zealand Clinical Trials Registry (Trial ID: ACTRN12621000031864).
This pilot feasibility trial was a sub-study of an ongoing longitudinal observational cohort investigation of individuals with confirmed SARS-CoV-2 infection led by St Vincent’s Hospital, Sydney Australia (ADAPT). The ADAPT study included individuals with COVID-19 disease, regardless of severity, who participated in a broad range of assessments to comprehensively characterize the immune-pathobiological effects of COVID-19 disease.21 Participants from the ADAPT cohort who reported ongoing symptoms, defined as persistent dyspnoea, fatigue or chest pain, at any time-point after 110 days post diagnosis were screened for inclusion in this pilot feasibility trial.
Participants
Participants in the ADAPT study were contacted via telephone by a member of the investigating team and were screened for eligibility. Participants were eligible for enrolment if they had i) consented to the ADAPT study, ii) were aged 16 years and over and iii) had ongoing symptoms of PCC, defined as persistent dyspnoea, fatigue and/or chest pain at any time beyond 4 months post SARS-Cov-2 diagnosis and not explained by an alternative diagnosis.22 Patients were excluded if they had i) pre-existing lung disease amenable to pulmonary rehabilitation eg COPD, ii) completed supervised exercise training in the 3- month period prior, iii) lived in a household where a member had already been recruited to this pilot feasibility trial, iv) were unable to participate due to other medical conditions and/or v) were unwilling to participate in telerehabilitation. The reason for declining participation was recorded. Consenting eligible participants underwent a standardised in-person baseline assessment during the enrolment visit.
Randomisation
After the enrolment visit, participants were randomised (1:1) using a computer generated randomisation (www.sealedenvelope.com) with concealed allocation to one of two groups: 1) telerehabilitation that included physiotherapist-supervised exercise training sessions via videoconferencing; and 2) a control group who performed no supervised exercise training over the study period and received usual care.
Interventions
Telerehabilitation Intervention Group
The telerehabilitation group exercise intervention was based on previous telerehabilitation programs that have been reported to be effective to increase physiological functional capacity in patients with respiratory limitations.23 Participants allocated to the intervention group were given instructions via email to set up a home-based exercise area and advised of the equipment requirements; a walking track free of clutter, weighted objects to use as hand weights and a stable chair. An instructional copy of the Borg dyspnoea scale was also provided along with links to join the Zoom videoconferencing platform. All telerehabilitation sessions were conducted by physiotherapists using the Zoom videoconferencing platform at St Vincent’s Private Hospital Sydney, in groups of up to six participants, twice weekly for ten weeks. Participants were screened by the physiotherapist prior to the commencement of each telerehabilitation exercise session to ensure safe participation and to identify any adverse events. Participants who failed to attend an exercise session were contacted by a member of the investigating team to ensure that no adverse event had occurred. An adverse event was defined as any physical and/or psychological condition that required consultation with the participants treating thoracic physician or primary care provider and/or precluded participation in a telerehabilitation exercise session. Adverse events that were identified were recorded by the treating physiotherapist and reported to the thoracic physician (AB) who was a member of the investigating team. Participants were required to complete at least 16 telerehabilitation exercise sessions to meet the exercise adherence definition and to allow for sessions missed due to illness or other commitments.
The 45 minute telerehabilitation exercise sessions consisted of i) warm up exercises (2 mins), ii) walking training (15 mins), iii) recovery break for hydration and breathing control (1 min), iv) unsupported upper limb exercises (5 mins), v) compound upper and lower limb exercises (15 mins) , vi) recovery break for hydration and breathing control (1 min), vii) Unsupported upper limb exercises (2 mins), viii) cool down (4 mins). Modifications were made to the components of the telerehabilitation exercise sessions so that participants were exercising at a rating of three to five on the modified Borg scale (0-10) for dyspnoea and perceived exertion. The detailed content of a telerehabilitation exercise session is presented in Supplement 1. Participants were monitored throughout each telerehabilitation exercise session by the physiotherapist for any signs of distress to ensure safe participation. During the two recovery breaks and cool down components of the telerehabilitation exercise sessions each participant was reviewed by the physiotherapist to ensure that they were no adverse events. Participants were not given any specific exercise prescription to complete on other days.
Control Group
Participants allocated to the control group received usual medical care and participation in other observational components of the larger longitudinal ADAPT study. The control group participants did not participate in supervised exercise training and were not given any advice regarding exercise training. The control group were given access to the same telerehabilitation exercise sessions or individual physiotherapist advice after completion of their control period.
Post-Intervention
All participants attended one in-person visit at enrolment and one at the completion of the study at St Vincent’s Private Hospital Sydney. At both in-person visits, all outcome measures were collected by physiotherapists who were blinded to the group allocation with no involvement in any components of the telerehabilitation exercise sessions. Participants in the trial and the physiotherapists who were blinded to the group allocation were instructed not to discuss the intervention received when undertaking the post-intervention outcome assessment procedures.
Outcome Measures
Feasibility Outcomes
The primary outcomes for this pilot feasibility trial were i) study recruitment rate, ii) adherence rate to the telerehabilitation program, iii) completion rate, iv) adverse events during the telerehabilitation program and v) technological issues limiting participant participation. The recruitment rate was defined as the number of patients that met the inclusion criteria who were then enrolled in the trial, divided by the number of patients contacted to be invited to the study. Adherence to the telerehabilitation program was measured by the percentage of enrolled participants who completed at least 16 of the 20 telerehabilitation exercise sessions. Completion was measured as the percentage of participants who completed the post-intervention outcome assessments. Safety was measured by the number of adverse events reported during the trial. Technological issues limiting connectivity to and participation in the telerehabilitation exercise sessions were also recorded.
Exploratory Clinical Outcomes: Exploratory clinical outcomes included i) six-minute walk test (6MWT), ii) HRQoL, iii) perceived level of fatigue, iv) five repetition sit to stand (5STS) test, v) self-selected gait speed and vi) handgrip grip strength.
Six-Minute Walk Test
Physiological functional capacity was estimated using the 6MWT. Two standardised 6MWTs were completed at least 30 minutes apart.24 The longest distance recorded from either of the two 6MWTs was used for analysis. The 6MWT result was compared to 6MWT estimates for healthy Australian individuals.25 Peripheral oxygen saturation (Sp02) and heart rate (HR) were continuously monitored during the 6MWT with a pulse oximeter (Masimo-Rad-5v, Masimo Corporation, Irvine, Ca, USA). Perceived level of dyspnoea was determined before and after completion of each 6MWT using the modified 0-10 Borg scale of perceived dyspnoea.26
Health Related Quality of Life
HRQoL was assessed by the St George’s Respiratory Questionnaire (SGRQ). The SGRQ rates the HRQoL of individuals with chronic respiratory disease on a 0-100 scale with a increasing scores indicating decreasing HRQoL.27
Perceived Level of Fatigue
The perceived level of fatigue was assessed using the 0–52-point FACIT-Fatigue scale, with a score of less than 34 points indicating severe levels of fatigue. 28,29
Five Repetition Sit to Stand Test
Lower limb functional capacity was measured using the 5STS test. Participants were asked to stand up and sit down from a 48cm chair, without using their arms, five times as fast as possible. The fastest time of the two 5STS tests was recorded for subsequent analysis. Participants who could not stand up without using their arms did not complete the 5STS test.30
Self-Selected Gait Speed
Self-selected gait speed was assessed using the four-metre gait speed test (4MGS), with the test completed three times using a standardised procedure. The lowest time recorded was used for subsequent analysis.31
Handgrip Strength
Handgrip strength was measured using a handgrip dynamometer (Jamar Plus Dynamometer, Cedaburg Wisconsin USA). Each participants hand was tested alternatively three times. The average result for the dominant hand was used for subsequent analysis.32
Sample Size
As a pilot feasibility trial, the objective was to test the trial procedures, recruitment potential and safety. The future trial arising from this pilot will aim for a medium effect size with 80% power; as such Bell et al.33 recommend 10 participants per arm in the pilot study.
Data Analysis
Demographic and symptom variables were summarized using means and standard deviations for numerical variables and count with percentage for categorical variables. Differences between groups were assessed using Wilcoxon rank-sum tests and Fisher’s exact tests due to the small sample sizes. For each exploratory clinical outcome measure, two-tailed two-sample t-tests were used to determine the difference between groups, and the difference in change in outcomes between groups, as per the protocol. Results are presented as mean difference with 95% confidence interval (CI), p-value and Cohen’s D effect size. All statistical analyses were performed using R version 4.2.2.34
Results
Flow of Participants
The flow of participants throughout the trial is presented in Figure 1. In March 2021 a total of 141 participants in the ADAPT longitudinal study were screened for PCC symptoms among whom there were 54 participants who met the inclusion criteria and were invited to participate in the pilot trial. A total of 21 out of the 54 (39%) participants consented to participate in our pilot feasibility trial.
Figure 1. Participant flow.
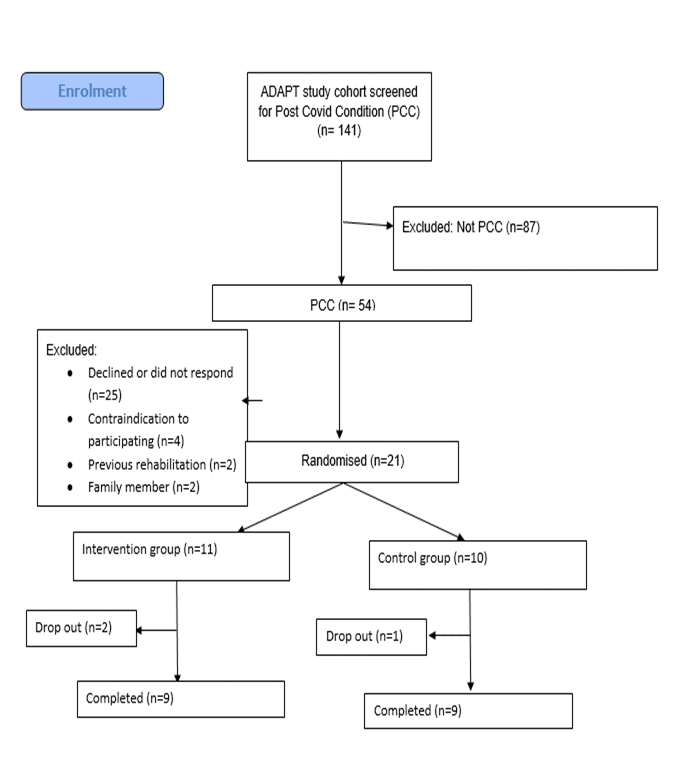
Among consenting participants, the average time between receiving a COVID-19 diagnosis and commencing the study was 365 ± 67 days since most ADAPT study participants had COVID-19 in March or April 2020. The majority of participants were managed in the community for the acute COVID-19 period, with only four (19%) requiring hospitalisation and of these one participant (5%) admitted to the intensive care unit. Eleven participants were randomised to the telerehabilitation physiotherapist-supervised exercise training group and ten to the control group. The study follow-up ended in August 2021 when all participants had completed the training protocol and had undergone a final assessment. The baseline characteristics of the participants are presented in Table 1.
Feasibility Outcomes
Adherence with the telerehabilitation exercise sessions was high for the participants who completed the study. The telerehabilitation group participants completed an average of 18 ±2 sessions, with 100% completing 16 sessions or more. There were two participants in the intervention arm of the trial who withdrew due to work and family commitments within one week of randomisation, attending two or less telerehabilitation exercise sessions. No adverse events were recorded during any of the telerehabilitation exercise sessions and no participants reported any severe post-exertional malaise that caused any modification to work or social activity after any of the exercise sessions. There were no adverse events recorded in the control group. There were only two minor technical issues reported during the telerehabilitation exercise sessions, both relating to poor audio quality from a participant’s device that was managed by the physiotherapist speaking to the participant over the telephone while viewing the participant via the computer screen.
Table 1. Baseline characteristics of patients
|
Overall(n=21) |
Telerehabilitation Group (n=11) |
Control Group (n=10 |
p-value |
Age (years) |
53 (15) |
59 (14) |
47 (13) |
0.034 |
Male; n (%) |
10 (48) |
6 (55) |
4 (40) |
0.670 |
BMI (kg/m2) |
26.4 (4.2) |
26.9 (3.8) |
25.9 (4.7) |
0.418 |
Lung function FEV1 L FEV1 % predicted FEV1/FVC% |
3.1 (0.7) 92 (19) 84 (13) |
3.1 (0.8) 96 (18) 78 (6) |
3.2 (0.7) 88 (21) 90 (15) |
0.622 0.291 0.005 |
Days since COVID-19 diagnosis |
366 (67) |
352 (80) |
380 (48) |
0.832 |
Hospitalisation for COVID-19 with or without an ICU admission; n (%) |
4 (19) |
3 (27) |
1 (10) |
0.587 |
Symptoms; n (%) Dyspnoea Fatigue Chest pain Cough Sputum |
18 (86) 16 (76) 8 (38) 8 (38) 2 (20) |
10 (91) 9 (82) 4 (36) 4 (36) 1 (9) |
8 (80) 7 (70) 4 (40) 4 (40) 1 (10) |
0.587 0.635 1.000 1.000 1.000 |
BMI: body mass index; FEV: forced expiratory volume; FVC: forced vital capacity; ICU: intensive care unit; Numerical variables presented as mean and standard deviation (SD); categorical variables presented as count with percentage (%). Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
Clinical Outcomes
The exploratory clinical outcome variables at enrolment and completion are presented in Tables 2 and 3. Analyses of the clinical outcome variables demonstrate wide variability. At baseline, 6MWT distance for the telerehabilitation exercise group was significantly lower than the control group (516m vs 634m; p=0.016) and the baseline predicted 6MWT distance values for the intervention and control groups were 76% and 87% respectively. The 5STS test values was within the normal range for most participants when adjusted for age.35 For the baseline 6MWT and 5STS values, the significant difference between the intervention and control groups (Table 2) suggests that future large studies could consider using an analysis of covariance approach where follow-up differences are adjusted for baseline measurement, though larger samples will likely result in better balance. There were no between group differences at follow-up (Table 2) or in change scores (Table 3) in any clinical variables measured aside from improvements in 5STS test values in favour of the intervention group (-1.4 seconds, 95% confidence interval -0.2 to -2.6). The 6MWT, 5STS test, FACIT fatigue scale and self-selected gait speed results suggest the potential for moderate to large effects to be found for both difference between groups at follow-up (Table 2) and difference in change between groups (Table 3) while differences in SGRQ were negligible.
Table 2. Clinical outcome summaries at baseline and follow-up with 95% confidence intervals (CI)
Baseline mean (95%CI) |
Follow-up mean (95%CI) |
|||||||
Outcome |
Exercise Group (n=11) |
Control Group (n=10) |
Difference |
P-value Cohen’s D |
Exercise Group (n=9) |
Control Group (n=9) |
Difference |
P-value Cohen’s D |
6MWT (m) |
516 (434, 598) |
634 (579, 690) |
-118 (-211, -25) |
0.016 -1.15 |
566 (445, 687) |
651 (592, 710) |
-85 (-212, 42) |
0.170 -0.69 |
Proportion of predicted 6MWT distance (%) |
76 (64, 88) |
87 (80, 94) |
-11 (-25, 2) |
0.083 -0.80 |
84(70, 99) |
89 (82, 97) |
-5 (-20, 10) |
0.510 -0.32 |
SGRQ-T |
27.9 (16.1, 39.7) |
20.3 (8.09, 32.5) |
7.6 (-8.2, 23.4) |
0.328 0.44 |
21.8 (8.93, 34.6) |
18.9 (6.16, 31.7) |
2.9 (-13.8, 19.5) |
0.721 0.17 |
SGRQ-S |
30.1 (15.7, 44.4) |
28.7 (12.8, 44.6) |
1.4 (-18.7, 21.3) |
0.889 0.06 |
21.6 (4.5, 38.7) |
25.6 (11.1, 40.2) |
-4.0 (-24.7, 16.6) |
0.686 -0.19 |
SGRQ-I |
19.5 (6.9, 32.1) |
13.5 (4.3, 22.7) |
6.0 (-8.7, 20.6) |
0.401 0.37 |
12.1 (1.4, 22.9) |
14.2 (1.6, 26.8) |
-2.1 (-17.3, 13.3) |
0.780 -0.13 |
SGRQ-A |
41.3 (27.5, 55.2) |
29.4 (11.1, 47.6) |
11.9 (-9.4, 33.4) |
0.255 |
39.0 (19.5, 58.5) |
25.8 (9.3, 42.3) |
13.2 (-10.3, 36.8) |
0.249 |
FACIT- Fatigue scale |
33.2 (25.9, 40.4) |
36.5 (28.7, 44.3) |
-3.3 (-13.2, 6.6) |
0.493 -0.31 |
39.9 (32.0, 47.8) |
38.3 (30.0, 46.6) |
1.6 (-9.0, 12.1) |
0.758 0.15 |
5STS (sec) |
8.6 (7.3, 10.0) |
6.5 (5.2, 7.8) |
2.1 (0.4, 3.9) |
0.020 1.14 |
6.8 (5.3, 8.3) |
5.9 (4.7, 7.1) |
0.9 (-0.8, 2.7) |
0.282 0.55 |
4m Gait speed (sec) |
2.9 (2.4, 3.3) |
2.5 (2.2, 2.8) |
0.4 (-0.1, 0.9) |
0.118 0.71 |
2.7 (2.2, 3.2) |
2.5 (2.2, 2.9) |
0.2 (-0.4, 0.7) |
0.645 0.22 |
Grip strength (kg) |
27.5 (18.1, 36.9) |
29.8 (25.1, 34.5) |
-2.3 (-12.3, 7.7) |
0.635 -0.21 |
29.0 (17.1, 40.9) |
31.2 (25.9, 36.4) |
-2.2 (-14.5, 10.3) |
0.713 -0.18 |
6MWT: six minute walk test in metres, SGRQ-T: St George’s Respiratory Questionnaire Total score, SGRQ-S: St George’s Respiratory Questionnaire Symptom score, SGRQ-I: St George’s Respiratory Questionnaire Impact score, SGRQ-A: St George’s Respiratory Questionnaire Activity score; 5STS: five repetition sit to stand.
Table 3. Difference in mean change between groups.
Outcome |
Exercise Group (n=11) |
Control Group (n=10) |
Difference(95%CI) |
P-value |
Cohen’s D |
6MWT (m) |
57 (-6, 120) |
6 (-10, 23) |
51 (-13, 114) |
0.108 |
0.84 |
Proportion of 6MWT predicted distance (%) |
0.07 (-0.01, 0.16) |
0.01 (-0.01, 0.03) |
0.06 (-0.02, 0.15) |
0.103 |
0.85 |
SGRQ-T |
-4.0 (-10.1, 2.0) |
-3.3 (-10.7, 4.2) |
-0.7 (-9.6, 8.1) |
0.854 |
-0.09 |
SGRQ-S |
-6.8 (-18.2, 4.6) |
-4.3 (-13.6, 5.1) |
-2.5 (-16.1, 11.1) |
0.703 |
-0.18 |
SGRQ-I |
-3.7 (-8.6, 1.2) |
-0.8 (-7.9, 6.3) |
-2.9 (-10.9, 5.1) |
0.452 |
-0.36 |
SGRQ-A |
-2.9 (-17.1, 11.3) |
-6.9 (-18.1, 4.4) |
4.0 (-12.8, 20.7) |
0.622 |
0.24 |
FACIT-Fatigue scale |
7.7 (1.1, 14.3) |
3.1 (0.8, 5.4) |
4.6 (-2.2, 11.3) |
0.164 |
0.71 |
5STS (sec) |
-1.8 (-2.8, -0.9) |
-0.4 (-1.3, 0.5) |
-1.4 (-2.6, -0.2) |
0.024 |
-1.20 |
4m Gait speed (sec) |
-0.3 (-0.7, 0.2) |
0.1 (-0.2, 0.4) |
-0.4 (-0.8, 0.1) |
0.128 |
-0.76 |
Grip strength (kg) |
2.4 (-0.9, 5.7) |
2.0 (-0.2, 4.1) |
0.4 (-3.2, 4.1) |
0.797 |
0.12 |
6MWT: six minute walk test in metres, SGRQ-T: St George’s Respiratory Questionnaire Total score, SGRQ-S: St George’s Respiratory Questionnaire Symptom score, SGRQ-I: St George’s Respiratory Questionnaire Impact score, SGRQ-A: St George’s Respiratory Questionnaire Activity score; 5STS: five repetition sit to stand.
Discussion
In this investigation we report the feasibility findings for a randomized controlled trial of a physiotherapist-supervised, group-based, telerehabilitation program delivered over 10 weeks for adults with PCC . Our preliminary data is encouraging for the design of larger trials to investigate the effects of a supervised group-based telerehabilitation intervention for the management of PCC symptoms. We report that the physiotherapist-supervised group-based telerehabilitation delivered to participants with PCC was feasible and safe. There were no adverse events, adherence was high with a recruitment rate consistent with previous telerehabilitation randomised controlled trials. 23,36 The exploratory results for our telerehabilitation intervention should be interpreted with caution due to the small sample size. However, the improvements in 6MWT and FACIT-Fatigue score for the intervention group, while not statistically significant, were encouraging and above the minimally important difference that have been reported for these measures 24,27 with moderate to large effects. Our results need to be replicated in a correctly powered sample and the responsiveness to change in these outcomes needs to be better understood in PCC.
PCC is a recognised condition that can have a prolonged and substantial impact on physiological functional capacity, fatigue, and HRQoL. PCC symptoms present after the acute COVID-19 infection period regardless of the severity of the acute COVID-19 symptom presentation.3 The health burden and impact of PCC occurs across all age groups and is not limited to those with more severe acute COVID-19 symptoms or comorbidities. Our participants had a wide age range, varying levels of baseline function and low rates of hospitalisation, while reporting ongoing fatigue, reduced HRQoL and reduced exercise capacity even though a year had elapsed since their COVID-19 diagnosis. The scale and variability of individuals with PCC presents challenges to planning and implementing effective and accessible rehabilitation programs. While clinical and research rehabilitation interventions earlier in the COVID-19 pandemic focussed on those patients with more severe disease in the acute period, 38–41 a greater understanding of individuals who experience persistent symptoms is required to reduce to impact of PCC including health care service delivery.
During the COVID-19 pandemic, hospital and rehabilitation services were limited, particularly in countries with high rates of disease. Resources were initially directed to patients and clinical services managing the acute COVID-19 presentations. As a result, many individuals with PCC did not have access to rehabilitation in a timely manner. Dedicated programs have now commenced to provide PCC rehabilitation services for those patients with symptoms longer than three months, with promising results.42,15 Inpatient and supervised outpatient rehabilitation programs for PCC have been shown to be feasible, 30–32,43,44 but these modes may not suit all individuals who present with PCC symptoms, including those who live far from health services and/or those with competing work and family commitments. Barriers to participation in inpatient and supervised rehabilitation services have been shown to reduce uptake in other disease groups.45,46 In order to increase the uptake and completion rates, rehabilitation services need to provide options to overcome these barriers for PCC individuals. Telerehabilitation provides a practical option for those individuals who cannot attend in person rehabilitation programs. PCC telerehabilitation programs have been previously reported including unsupervised home programs with telephone or phone app support, 47 individual telehealth sessions 48 and a virtual service.49 As PCC has severely impacted some individuals’ ability to return to their previous occupation,2 telerehabilitation allows access for people who need to schedule services outside of their work commitments. Our study included an after-hours option for those participants who required such flexibility. Dalbosco-Salas et al 16 trialled a similar telerehabilitation model to our study in 115 people on average 30 days after COVID-19 infection with significant improvements in exercise capacity, HRQOL, fatigue and dyspnoea. However, our study is novel due to the inclusion of a control group and including participants who were on average one year from their original COVID-19 diagnosis. We report that telerehabilitation is feasible, safe and potentially effective for those who have experienced PCC symptoms for a longer duration and were unable to access rehabilitation earlier.
To our knowledge, our trial is the first to report the feasibility of a group-based telerehabilitation intervention in PCC. Our exercise intervention had minimal equipment requirements and adherence was high. However, it is important to note that all of our participants had access to, and regularly used videoconferencing platforms prior to the commencement of our feasibility trial. As a result, we encountered less technological issues compared to previous telerehabilitation studies 23,24 and fewer barriers to uptake of the exercise intervention. Additionally, prior to the commencement of our feasibility trial, all participants were medically stable and had undergone extensive medical investigations such as pulmonary function testing and cardiac investigations, which may have contributed to no adverse events reported during the trial.
Our study design has several limitations that should be considered when planning larger randomised controlled clinical trials. The exercise and control groups were heterogenous with wide variability in baseline physiological functional capacity, fatigue, and HRQoL measures. The wide variability in baseline clinical outcome measures suggests the need for statistical analyses that adjust for baseline (analysis of covariance) in larger randomised trials. Larger randomised controlled trials can potentially investigate the effect of different types of rehabilitation interventions and the effect of telerehabilitation for PCC participants who report different symptom presentations, such as those with breathlessness and/or fatigue. Individuals with PCC symptoms have also reported post-exertional malaise and conditions including postural orthostatic tachycardia.50 Although these conditions were not reported by our participants, there may be a subgroup of PCC patients who may not be suitable for this type of rehabilitation program. 51 In order to ensure that the control group was unable to independently replicate the content of the telerehabilitation exercise sessions, we excluded potential participants who were members of the same household and/or family of previously recruited participants. Since household transmission of COVID-19 is common, 52 there is the possibility of family members being eligible for the same randomised controlled clinical trials. Future randomised trials may need to adapt their randomisation protocols to manage this issue to ensure participants in the same household do not participate in different arms of the trial. Unlike previous telerehabilitation studies, 23,24 we did not complete a home-visit to set-up each participant’s exercise area. A home visit with each participant would have provided the opportunity for greater monitoring of the exercise intervention and progression of the program. Exercise prescription was based solely on the intensity of effort as indicated by shortness of breath with exertion. This provided a practical solution for monitoring the exercise intervention. However, closer monitoring of the intensity of effort for example the actual distance that was walked by participants during the exercise intervention may be beneficial in larger trials that evaluate the efficacy of different rehabilitation exercise interventions.
The clinical outcome measures used in this study are similar to those reported elsewhere.40,42,47 However the responsiveness to change of these clinical outcome measures needs to be further evaluated in PCC patients due to the wide variability of symptom presentations. Our participants had a high baseline 6MWT and 5STS test relative to other trials 40,42,47 despite reporting significantly reduced HRQoL. Consequently tests such as the 6MWT may not have been sensitive to change following the exercise intervention compared to other tests such as the endurance shuttle walk test (ESWT) or one minute sit to stand test. Investigations in larger PCC cohorts is required to identify the most appropriate clinical outcome measures following exercise based interventions.
In conclusion, our pilot feasibility trial has shown that a group-based physiotherapist-supervised telerehabilitation exercise program is feasible and safe for individuals with PCC. Further studies are required to evaluate the efficacy of the telerehabilitation exercise intervention for individuals with PCC.
Ethics Approval
The study was approved by the Sydney Local Health District Human Research Ethics Committee (X20-0545 & 2020/ETH03228).
Funding
This was a researcher-initiated study that was supported by funding from St Vincent’s Private Allied Health Services, The St Vincent’s Curran Foundation and The St Vincent’s Clinic Foundation.
Acknowledgements
Advice for the preparation of the manuscript was received from Amanda Thomas. Ashlea Hills participated in the delivery of the telerehabilitation exercise sessions.
Author Contributions
This study was initially conceived by MFK, AB and SFM with study design completed by MFK, AB, SFM, LD, and MP. BD, PdT, ARS, EK, ARP and EMB were involved in protocol development, data collection and delivery of the intervention. PLG conducted the statistical analyses. MFK completed the first draft of the manuscript with all authors reviewing and approving the final version.
Trial Registration
Australian New Zealand Clinical Trials Registry (Trial ID: ACTRN12621000031864).
Supplement 1
Supplement 1: Exercise Session Content
The 45-minute telehealth exercise sessions consisted of i) Warm up exercises (2 mins) ii) Walking training (15 mins) iii) Recovery break for hydration and breathing control (1 min) iv) Unsupported upper limb exercises (5 mins) v) Compound upper and lower limb exercises with modifications made so that the participants were exercising at a rating of three to five on the modified Borg scale (0-10) for dyspnoea and perceived exertion (15 mins) vi) Recovery break for hydration and breathing control (1 min) vii) Unsupported upper limb exercises (2 mins) viii) Cool down (4 mins)
Exercise Session Content |
Duration |
Content Description |
Warm up |
2 minutes |
Marching on spot, small squats, arm stretches |
Walking |
15 minutes |
10 laps of available walking space + 10 sit to stand exercises, repeated for the duration with intensity encouraged to remain 3-4/10 on the modified dyspnoea or rate of perceived exertion scale. Small dumbbells held in hands to increase intensity if needed |
Recovery Break |
1 minute |
Hydration and breathing control |
Unsupported upper limb exercises |
5 minutes |
Two sets of 10 repetitions each of three exercises using small dumbbells or water bottles |
Compound upper and lower limb exercises |
15 minutes |
1 minute each of compound aerobic exercises such as: mini squats with shoulder press, boxing combinations, lunges with lateral arm raises, heel digs with forward arm raise, marching on spot while boxing, heel raises with arm movements. Exercises modified to achieve 3-4/10 dyspnoea or rate of perceived exertion eg changing speed of exercises to make easier or harder or adding jumping components. |
Recovery Break |
1 minute |
Hydration and breathing control |
Unsupported upper limb exercises |
2 minutes |
Two sets of 10 repetitions of bicep curls and shoulder presses using small dumbbells or water bottles |
Cool down |
4 minutes |
Gentle stretching and breathing control |
References
- Organisation, W.H. WHO Coronavirus (Covid-19) dashboard. 2023 [cited 2021 November 25]; Available from: https://covid19.who.int/.
- Wu, T., Zuo, Z., Kang, S., et al., Multi-organ Dysfunction in Patients with COVID-19: A Systematic Review and Meta-analysis. Aging Dis, 2020. 11(4): 874–894.
- Evans, R.A., McAuley, H., Harrison, EM., et al., Physical, cognitive, and mental health impacts of COVID-19 after hospitalisation (PHOSP-COVID): a UK multicentre, prospective cohort study. Lancet Respir Med, 2021. 9(11): 1275–1287.
- Nalbandian, A., Sehgal, A., Gupta, A., et al., Post-acute COVID-19 syndrome. Nat Med, 2021. 27(4): 601–615.
- Parotto, M., Gyöngyösi, M., Hoiwe, K., et al., Post-acute sequelae of COVID-19: understanding and addressing the burden of multisystem manifestations. Lancet Respir Med, 2023. 11(8): 739–754.
- Goërtz, YMJ., Van Herck, M., Delbressine, JM., et al., Persistent symptoms 3 months after a SARS-CoV-2 infection: the post-COVID-19 syndrome? ERJ Open Research, 2020: 0542–2020.
- Stavem, K., Ghanima, W., Olsen, MK., et al., Persistent symptoms 1.5–6 months after COVID-19 in non-hospitalised subjects: a population-based cohort study. Thorax, 2021. 76(4): 405–407.
- Huang, C., Huang, L., Wang, Y., et al., 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. The Lancet, 2021. 397(10270): p. 220–232.
- Morin, L., Savale, L., Pham, T., et al., Four-Month Clinical Status of a Cohort of Patients After Hospitalization for COVID-19. JAMA, 2021. 325(15): 1525–1534.
- Darley, D.R., Dore, DJ., Cysique, L., et al., Persistent symptoms up to four months after community and hospital-managed SARS-CoV-2 infection. Med J Aust, 2021. 214(6): p. 279–280.
- Denehy, L. and Puthucheary, Z. Surviving COVID-19: a familiar road to recovery? Lancet. Respir Med, 2021. 9(11): 1211–1213.
- McCarthy, B., Casey, D., Devane, D., et al., Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev, 2015(2).
- Dowman, L., Hill, CJ., May, A., et al. Holland, Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev, 2021(2).
- Cox, N.S., Dal Corso, D., Hansen, H., et al., Telerehabilitation for chronic respiratory disease. Cochrane Database Syst Rev, 2021, 1(1): CD013040.
- Ostrowska, M., Rzepka-Cholasińska, A., Pietrzykowski, L., et al., Effects of Multidisciplinary Rehabilitation Program in Patients with Long COVID-19: Post-COVID-19 Rehabilitation (PCR SIRIO 8) Study. J Clin Med, 2023. 12(2).
- Dalbosco-Salas, M., Torres-Castro, R., Rojas Leyton, A., et al., Effectiveness of a Primary Care Telerehabilitation Program for Post-COVID-19 Patients: A Feasibility Study. J Clin Med, 2021. 10(19): 4428.
- Spruit, M.A., Holland, A., Singh, SJ., et al., COVID-19: Interim Guidance on Rehabilitation in the Hospital and Post-Hospital Phase from a European Respiratory Society and American Thoracic Society-coordinated International Task Force. Eur Respir J, 2020. 56(6).
- Singh, S.J., Baldwin, MM., Daynes, E., et al., Respiratory sequelae of COVID-19: pulmonary and extrapulmonary origins, and approaches to clinical care and rehabilitation. Lancet Respir Med, 2023. 11(8): 709–725.
- Eldridge, S.M., Chan, CL., Campbell, MJ., et al., CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ, 2016. 355: i5239.
- Hoffmann, T.C., Glasziou, PP., Boutron, I., et al., Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ: Brit Med J, 2014. 348: g1687.
- Darley, D., Dore, GJ., Byrne, AL., et al., Limited recovery from post-acute sequelae of SARS-CoV-2 (PASC) at eight months in a prospective cohort. medRxiv, 2021: 2021.03.29.21254211.
- Soriano, J.B., Murthy, S., Marshall, JC., et al., A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect Dis, 2022. 22(4): e102–e107.
- Tsai, L.L.Y., McNamara, RJ., Moddel, C., et al., Home-based telerehabilitation via real-time videoconferencing improves endurance exercise capacity in patients with COPD: The randomized controlled TeleR Study. Respirol, 2017. 22(4): 699–707.
- Holland, A.E., Spruit, MA., Troosters, T., et al., An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J, 2014. 44(6): 1428–1446.
- Jenkins, S., Cecins, N., Camarri, B., et al., Regression equations to predict 6-minute walk distance in middle-aged and elderly adults. Physiother Theory Pract, 2009. 25(7): 516–522.
- Borg, G.A., Psychophysical bases of perceived exertion. Med Sci Sports Exerc, 1982. 14(5): 373–381.
- Jones, P.W., F.H. Quirk, and C.M. Baveystock, The St George's Respiratory Questionnaire. Respir Med, 1991. 85 Suppl B: p. 25–31; discussion 33–37.
- Yellen, S.B., Cella, DF., Webster, K., et al., Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. J Pain Symptom Manage, 1997. 13(2): 63–74.
- Van Belle, S., and Kerger, J., Comparison of proposed diagnostic criteria with FACT-F and VAS for cancer-related fatigue: proposal for use as a screening tool. Support Care Cancer, 2005. 13(4): 246–254.
- Jones, S.E., Kon, SS., Canavan, JL., et al., The five-repetition sit-to-stand test as a functional outcome measure in COPD. Thorax, 2013. 68(11): 1015–1020.
- Kon, S.S., Patel, MS., Canavan, JL., et al., Reliability and validity of 4-metre gait speed in COPD. Eur Respir J, 2013. 42(2): 333–340
- Karagiannis, C., Savva, C., Korakakis, V., et al., Test-Retest Reliability of Handgrip Strength in Patients with Chronic Obstructive Pulmonary Disease. Copd, 2020. 17(5): 568-574.
- Bell, M.L., Whitehead, AL, and Julious, SA. Guidance for using pilot studies to inform the design of intervention trials with continuous outcomes. Clin Epidemiol, 2018. 10: 153–157.
- Team, R.C., A Language and Environment for statistical computing. 2021, R Foundation for Statistical Computing: Vienna, Austria.
- Bohannon, R.W., Bubela, DJ., Magasi, SR., et al., Sit-to-stand test: Performance and determinants across the age-span. Isokinet Exerc Sci, 2010. 18(4): 235–240.
- Wootton, S.L., Ng, JWC., McKeough, ZJ., et al., Ground-based walking training improves quality of life and exercise capacity in COPD. Eur Respir J, 2014. 44(4): 885–894.
- Rebelo, P., Oliveira, A., Andrade, L., et al., Minimal Clinically Important Differences for Patient-Reported Outcome Measures of Fatigue in Patients With COPD Following Pulmonary Rehabilitation. CHEST, 2020. 158(2): 550–561.
- Wootton, S.L., King, M., Alison, JA., et al., COVID-19 rehabilitation delivered via a telehealth pulmonary rehabilitation model: a case series. Respirol Case Rep, 2020. 8(8): e00669.
- Betschart, M., Rezek. S., Unger, I., et al., Feasibility of an Outpatient Training Program after COVID-19. Int J Environ Res Public Health, 2021. 18(8).
- Gloeckl, R., Leitl, D., Jarosch, I., et al., Benefits of pulmonary rehabilitation in COVID-19: a prospective observational cohort study. ERJ Open Res, 2021. 7(2).
- Szarvas, Z., Fekete, M, Horvath, R., et al., Cardiopulmonary rehabilitation programme improves physical health and quality of life in post-COVID syndrome. Ann Palliat Med, 2023. 12(3): 548–560.
- Daynes, E., Gerlis, C., Chaplin, E., et al., Early experiences of rehabilitation for individuals post-COVID to improve fatigue, breathlessness exercise capacity and cognition – A cohort study. Chron Respir Dis, 2021. 18:14799731211015691.
- Mammi, P., Ranza, E., Rampello, A., et al., Post-COVID-19 Ongoing Symptoms and Health-Related Quality of Life: Does Rehabilitation Matter?: Preliminary Evidence. Am J Phys Med Rehabil, 2023. 102(3): 241–244.
- Barbara, C., Clavario , P., De Marzo, V., et al., Effects of exercise rehabilitation in patients with long coronavirus disease 2019. Eur J Prev Cardiol, 2022. 29(7): e258–e260.
- Hayton, C., Clark, A., Olive, S., et al., Barriers to pulmonary rehabilitation: Characteristics that predict patient attendance and adherence. Respir Med, 2013. 107(3): 401–407.
- Keating, A., Lee, A., and Holland, AE. What prevents people with chronic obstructive pulmonary disease from attending pulmonary rehabilitation? A systematic review. Chron Respir Dis, 2011. 8(2): 89–99.
- Li, J.a., Xia, W., Zhan, C., et al., A telerehabilitation programme in post-discharge COVID-19 patients (TERECO): a randomised controlled trial. Thorax, 2021: p. thoraxjnl-2021; 217382.
- Vallier, J.M., Simon, C., Bronstein, A., et al., Randomized controlled trial of home-based vs. hospital-based pulmonary rehabilitation in post COVID-19 patients. Eur J Phys Rehabil Med, 2023. 59(1): 103-110.
- Flannery, T., Brady-Sawant, H., Brady-Sawant, R., et al., A Mixed-Methods Evaluation of a Virtual Rehabilitation Program for Self-Management in Post-COVID-19 Syndrome (Long COVID). Int J Environ Res Public Health, 2022. 19(19).
- Twomey, R., DeMars, J., Franklin, K., et al., Chronic fatigue and post-exertional malaise in people living with long COVID. medRxiv, 2021: 2021.06.11.21258564.
- Physiotherapy, W., World Physiotherapy Response to Covid-19 Briefing Paper 9. Safe rehabilitation approaches for people living with Long Covid: physical activity and exercise. 2021, World Physiotherapy: London.
- Madewell, Z.J., Yang, Y., Longini Jr, IM., et al., Household Transmission of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Netw Open, 2020. 3(12): e2031756.



