Home / Journal / Annals of Cardiology
Multiple Arrhythmias in COVID-19 Patients: A Case Series
COVID-19 ventricular tachycardia myocardial injury intermittent bradycardia cardiac arrhythmia atrial fibrillation
Savalan Babapoor-Farrokhran, Zachary Port, Deanna Gill, Zaid Ammari, Sumeet Mainigi, and Aman Amanullah
DOI: 10.31532/AnnCardiol.1.1.002 20 Oct 2020
Abstract
Extra-pulmonary manifestations of severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] infection such as cardiovascular complications have been reported and previous case reports have documented evidence of myocardial damage. Currently, very few case reports of the arrhythmias associated with Coronavirus Disease 2019 [COVID-19] exist.
In this case series, we demonstrate two cases of critically ill COVID-19 patients with cardiac rhythm abnormalities that might be a direct or indirect consequence of this infection. Each of the patients experienced a variety of cardiac rhythm abnormalities. None of the patients had any record of cardiac rhythm abnormalities prior to admission. The unique point of these cases is the multiple dysrhythmias in the same patients that have not been reported previously.
We demonstrated that critically ill COVID-19 patients develop different arrhythmias ranging from sinus tachycardia, bradycardia, atrial fibrillation, supraventricular tachycardia, and non-sustained ventricular tachycardia. Non-sustained monomorphic ventricular tachycardia was the most common arrhythmia in the two cases despite normalization of electrolyte imbalances and avoidance of QTc prolonging agents.
Keywords
COVID-19, ventricular tachycardia, myocardial injury, intermittent bradycardia, cardiac arrhythmia, atrial fibrillation
Introduction
The current outbreak of Coronavirus Disease 2019 [COVID-19] caused by the virus severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] has quickly emerged as a global health crisis. As of 11 May 2020, over 4 million people worldwide have been infected with the virus since its initial outbreak in Wuhan, China in December 2019.1
While the respiratory system is the organ system primarily affected by COVID-19, it also has extra-pulmonary manifestations affecting the gastrointestinal and cardiovascular systems as well.2,3 Some of the known cardiovascular complications of COVID-19 include myocardial damage, myocarditis, acute heart failure, acute coronary syndrome resulting from coronary artery thrombosis or plaque rupture.3 Cardiovascular complications have been well established with COVID-19. Case reports from previous outbreaks caused by members of the coronavirus family have cited manifestations of cardiac involvement resulting in myocardial damage and arrhythmias.4,5
Sinus tachycardia is the most commonly identified arrhythmia; however, other arrhythmias have also been reported at a significant rate in COVID-19 patients, including atrial arrhythmias, atrial fibrillation, sinus bradycardia, and ventricular arrhythmias.6,7,8
However, there are a limited number of documented case reports highlighting the relationship between COVID-19 and cardiac arrhythmias. In this report, we demonstrate two cases of COVID-19 with rhythm abnormalities that might be a direct or indirect consequence of this infection.
Case Presentation
Case 1: Ventricular Tachycardia, Supraventricular tachycardia, and Intermittent Bradycardia
A 59-year-old man with a history of type II diabetes, hypertension, hyperlipidemia, obstructive sleep apnea, stroke, and known SARS-CoV-2 exposure presented to the hospital from a nursing home with one day of worsening shortness of breath, fever, and hypoxia. On presentation, the patient was febrile at 40.9°C, saturating at 82% on room air, blood pressure of 116/64 mmHg and heart rate of 105 bpm. Laboratory tests demonstrate a creatinine of 2.0 mg/dL, potassium of 5.3 mmol/L (normal ref. 3.6-5.1 mmol/L), magnesium of 2.5 mg/dL (normal ref 1.6-2.6 mg/dL), troponin I of 0.81 ng/mL (normal ref. <0.03 ng/ml), brain natriuretic peptide [BNP] of 4,856 pg/mL (normal ref. <100 pg/ml), C-reactive protein of 233 mg/L (normal ref. 0.0-5.0 mg/L), procalcitonin of 11 ng/mL, and D-dimer 3,720 ng/mL (normal ref. 270-490 ng/ml). Admission electrocardiogram [ECG] demonstrated sinus rhythm with premature ventricular complexes and bigeminy (Figure 1A). Chest X-ray was consistent with multifocal pneumonia. He was given cefepime and vancomycin for empiric treatment of pneumonia. He was administered 6 liters/min of oxygen via high-flow nasal cannula with improved saturations. Despite this, his oxygen saturations improved to 84%, thus the patient was intubated at this time for hypoxic respiratory failure. At the time of his intubation his Horowitz Index was 74 mmHg/100% = 74mmHg, indicating severe acute respiratory distress syndrome [ARDS]. He was hypotensive and did not respond to 1.5L of normal saline and was started on inotropic support with norepinephrine. A central venous catheter was inserted and he was admitted to the intensive care unit [ICU]. On hospital day 1 in the ICU a bedside echocardiogram showed global hypokinesis with an ejection fraction of 10% and four chamber dilation. He was then found to be SARS-CoV-2 positive and azithromycin was given to treat potential atypical pneumonia. However, due to prolonged corrected QT interval [QTc] of 534 ms he only received one dose. His troponin peaked at 2.5 ng/mL and he was started on continuous heparin infusion with a goal partial thromboplastin time of 76-112 seconds, due to the concern for thromboembolic events that have typically been reported in COVID-19. His work of breathing increased with decreasing oxygen saturations and he was intubated. Cardiology consultation was obtained due to the elevated troponin level and recommended continued management of the infection, however, did not recommend a left heart catheterization at the time due to the patient’s critical state. On hospital day 2 the patient had his first episode of non-sustained wide complex tachycardia lasting 16 seconds and hypotension that self-resolved. His electrolytes were within normal limits the day of this episode. He subsequently developed multiple episodes of non-sustained ventricular tachycardia followed by supraventricular tachycardia (Figure 1B). On hospital day 3 the patient had three more episodes of self-limiting wide complex tachycardia. He became hypotensive requiring administration of vasopressin, phenylephrine and norepinephrine to maintain blood pressures as well as amiodarone drip to suppress dysrhythmia. No cardioversion was conducted due to self-limiting arrhythmia. Also, on hospital day 3 he developed intermittent bradycardia that resolved spontaneously. The patient continued to deteriorate while being on norepinephrine 12 mcg/min. Palliative care was consulted, and his condition was discussed with the family. Due to the patient’s end stage illness, it was decided to transition the patient to comfort care. On hospital day 12, the patient expired.
Figure 1. Representative electrocardiogram and telemetry strips of the patient in case 1. (A) Admission electrocardiogram of the patient demonstrating sinus rhythm with premature ventricular complexes and bigeminy; Right axis deviation; Nonspecific intraventricular conduction block; QTC: 534 ms. (B) Telemetry strips of the patient demonstrating non-sustained ventricular tachycardia, non-sustained ventricular tachycardia followed by SVT, and idioventricular rhythm. Printing speed for the ECG: 25 mm/s.
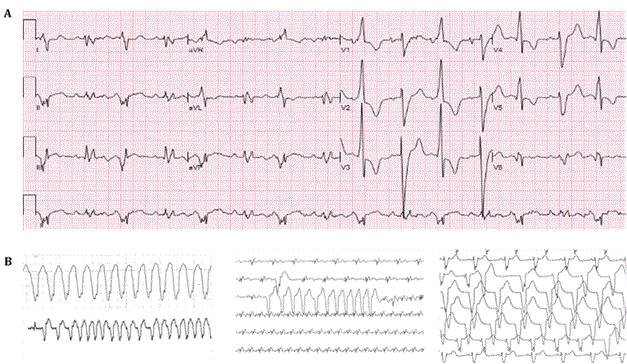
Table 1: Patient 1 Timeline
Time |
Events |
Hospital Day 0 |
Patient presented with hypoxia, fever, and shortness of breath. Admission EKG showed sinus tachycardia with premature ventricular beats and bigeminy (Figure 1A). |
Hospital Day 1 |
ECHO showed global hypokinesis with EF of 10% and four-chamber dilation. COVID-19 test resulted positive. |
Hospital Day 2 |
Episode of non-sustained wide complex monomorphic tachycardia lasting 16 seconds followed by multiple episodes of non-sustained ventricular tachycardia and then supraventricular tachycardia (Figure 1B). |
Hospital Day 3 |
Three more episodes of wide complex tachycardia resulting in hypotension requiring pressors. Developed intermittent bradycardia that resolved spontaneously |
Case 2: New Onset Atrial Fibrillation, Bradycardia, and Ventricular Tachycardia
A 75-year-old man with a past medical history of heart failure, stage III chronic kidney disease, human immunodeficiency virus [HIV] on antiretroviral therapy, and a history of cocaine abuse presented from a nursing home for hypoxia of 85% on room air and altered mental status. The patient tested positive for SARS-CoV-2 and was started on hydroxychloroquine two days prior to admission. On admission he was febrile at 40.7°C, hypoxic at 84% on room air, normotensive, with a heart rate of 119 bpm. Due to profound hypoxia and altered mental status the patient was intubated in the emergency department. His initial Horowitz Index was 86 mmHg/100% = 86 mmHg, indicating severe ARDS. Laboratory findings revealed a creatinine of 6.3 mg/dL, potassium of 5.1 mmol/L, magnesium of 3.1 mg/dL, troponin I of 0.8 ng/ml, C-reactive protein of 233 mg/L (normal ref. 0.0-5.0 mg/L), procalcitonin of 18 ng/mL, and a D-dimer >25,000 ng/mL. Chest X-ray showed bilateral airspace opacities concerning for pneumonia. ECG revealed sinus tachycardia with premature ventricular complexes and QTc of 419 ms (Figure 2A). The patient was admitted to the ICU with acute respiratory distress syndrome and started on broad spectrum IV antibiotics including cefepime and vancomycin. On hospital day 1, the patient had hypotension that did not respond to fluid administration and norepinephrine was administered. He was also found to be in atrial fibrillation with heart rate of 70 bpm to 90 bpm during this episode and amiodarone was given with minimal response. On hospital days 2-4 the patient’s temperatures improved and he remained afebrile but still required intermittent pressor support. On day 4 the hydroxychloroquine course was completed. On hospital days 5-10 the patient remained afebrile. The team attempted to wean him off of the ventilator and vasopressors with some success. He underwent continuous venovenous hemodialysis to maintain fluid balance and normal electrolyte levels. On hospital day 11 the patient developed atrial fibrillation with rapid ventricular response to 120 bpm requiring IV push metoprolol with good response (Figure 2B). The patient was also started on heparin infusion (goal partial thromboplastin time of 76-112 seconds) given his elevated thromboembolic risk. Frequent premature atrial contractions and non-sustained ventricular tachycardia were noted on telemetry (Figure 2C). The patient also had an increased fraction of inspired oxygen [FiO2] requirement on the ventilator. A repeat chest X-ray showed persistent multifocal infiltrates consistent with COVID pneumonia that was unchanged. The patient continued to have persistent hypotension through hospital day 12 requiring vasopressors and was unable to be weaned off from the ventilator. He also developed intermittent self-limiting bradycardia (Figure 2C). He had persistent episodes of atrial fibrillation with rapid ventricular response that were controlled with metoprolol (Figure 2B). Cardiology was consulted for recommendation regarding worsening clinical status and evaluation for mechanical circulatory support. He was deemed a poor candidate for extracorporeal membrane oxygenation [ECMO] due to his very poor prognosis. On hospital day 13 the patient had pulseless electrical activity arrest despite being on norepinephrine 50mcg/min, vasopressin 0.03 unit/min, and phenylephrine 300 mcg/min. Resuscitation was attempted following an advanced cardiac life support protocol, however, attempts were unsuccessful, and the patient expired.
Figure 2. Representative electrocardiograms and telemetry strips of the patient in case 2. (A) Admission electrocardiogram of the patient demonstrating sinus tachycardia with premature ventricular complexes; Left axis deviation; Inferior and anteroseptal infarct, age undetermined; QTC: 419 ms. (B) Electrocardiogram demonstrating atrial fibrillation. (C) Telemetry strips of the patient demonstrating non-sustained ventricular tachycardia, atrial fibrillation and bradycardia. Printing speed for the ECG: 25 mm/s
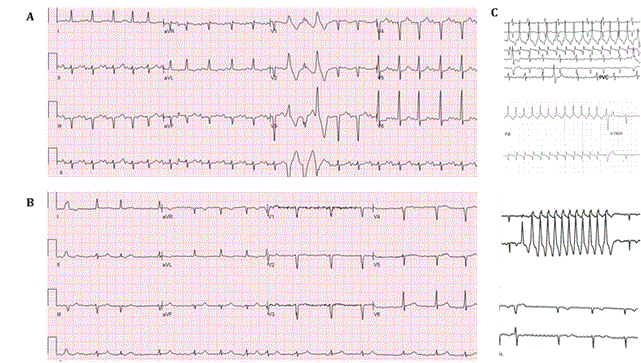
Table 2: Patient 2 Timeline
Time |
Events |
2 Days Prior to Admission |
Patient tested positive for COVID-19 and was started on hydroxychloroquine |
Hospital Day 0 |
Presented with hypoxia and altered mental status. EKG revealed sinus tachycardia with premature ventricular complexes and QTC of 419 ms (Figure 2A) |
Hospital Day 1 |
Went into atrial fibrillation with rapid ventricular response. Became hypotensive requiring pressor support. |
Hospital Days 2-4 |
Required intermittent pressor support. Hydroxychloroquine course complete. |
Hospital Days 5-10 |
Attempts to wean from pressors complicated by metabolic derangements |
Hospital Day 11 |
Again, developed atrial fibrillation with rapid ventricular response (Figure 2B). Repeated premature atrial contractions and non-sustained ventricular tachycardia were noted on telemetry (Figure 2C). |
Hospital Day 12 |
Continued episodes of atrial fibrillation. |
Hospital Day 13 |
Intermittent bradycardia (Figure 2C). |
Hospital Day 14 |
Patient expired |
Discussion
The majority of patients presenting with COVID-19 requiring hospital admission will not have signs or symptoms of arrhythmias or cardiac conduction system disease. Tachycardia can be seen in the setting of fever, shortness of breath, pain, etc. Liu et al. reported that about 7 percent of patients report palpitations as a presenting symptom.9,10 In a report from Wuhan, China, 16.7% of hospitalized and 44.4% of ICU patients with COVID-19 had cardiac arrhythmias.11 Additionally, a limited number of other studies have suggested an association with COVID-19 and other cardiac arrhythmias.9,12 Recent studies have suggested that myocardial injury can occur in COVID-19 patients especially in critically ill individuals via different mechanisms, mainly through direct damage to the cardiomyocytes, systemic inflammation, and hypoxia. 3,9 Whether the underlying mechanism of cardiac arrhythmias in COVID-19 is a result of myocardial damage, or the virus itself has a direct arrhythmogenic effect is not yet established. All in all, large scale studies are needed to explore the mechanisms of arrhythmias.
As evidenced by the studies conducted on critically ill COVID-19 patients, the prevalence of arrhythmias is high especially in those with evidence of myocardial injury or myocardial ischemia.6,9,10 Moreover, higher prevalence of arrhythmias can be seen in patients with electrolyte disturbances (eg, hypokalemia), acid-base imbalance, hypoxia, septic shock, cardiogenic shock, or evidence of diffuse systemic inflammation. It can also be seen in patients who are receiving QT-prolonging therapies. 6,9
In case 1, the initial troponin elevation of 0.81 ng/mL did not seem to correlate with the degree of global 4-chamber hypokinesis seen on the patient's echocardiogram. Additionally, the initial ECG was not suggestive of any type of regional ischemia. These two findings taken together in the clinical context make any type of regional ischemia, normally seen in coronary occlusion or even in cases of myocardial infarction with non-obstructive coronary arteries [MINOCA] unlikely. In case 2, the initial troponin of 0.80 ng/mL which peaked at 1.39 ng/mL, and an ECG not suggestive of acute ischemia do not indicate that coronary occlusion was the cause of the cardiac arrhythmias. The type of cardiac injury in these two cases can be classified as non-ST-elevation myocardial infarction. Taken together, these two cases suggest that the COVID-19 infection and its complications were the cause of the acute myocardial injury and dysfunction rather than underlying occult coronary artery disease. Unfortunately, due to the acute decompensation as well as the critical state of the patients on admission, a thorough cardiac workup including left heart catheterization or electrophysiology study were not able to be performed.
In these two case reports, we demonstrated that critically ill patients develop different arrhythmias ranging from sinus tachycardia, to sinus bradycardia, atrial fibrillation, supraventricular tachycardia, and non-sustained ventricular tachycardia. What is especially interesting is that each of these two cases had three of the above-mentioned arrhythmias which are not usually seen simultaneously in non-COVID-19 cases.
These arrhythmias have different mechanisms of initiation and propagation and their presence in a single individual reflects that the arrhythmogenic cause cannot be attributed to a single underlying pathophysiological mechanism. One possible mechanism of arrhythmogenesis concerns catecholamine levels: critically ill ICU-patients often require high levels of vasopressor support including norepinephrine, phenylephrine, and vasopressin. All of these are well-known to have arrhythmogenic potential themselves. 12,13 In these two cases, COVID-19 is the most possible etiology given the lack of co-occurrent variables and the multiple simultaneous arrhythmias which are generated by multiple pathophysiological mechanisms. We observed that non-sustained monomorphic ventricular tachycardia followed by intermittent bradycardia (the etiology of which is still unknown) was the most common arrhythmia in both cases despite normalization of electrolyte imbalances and avoidance of QTc prolonging agents. Other possible etiologies of cardiac arrhythmias for case 1 include prolonged QTc, myocarditis, septic shock, acid base imbalance, and electrolyte imbalance. This patient’s electrolytes were maintained within normal limits and acid-base disturbances were corrected, making these etiologies unlikely. Additionally, he was treated with empiric antibiotics that are not known to increase QTc. In case 2, COVID-19 is the most likely etiology given the normal QTc and electrolytes. The limitation of this case series is that patients were in critical condition and the duration of the admission was too short to investigate the pathophysiological mechanisms of their arrhythmias. Also, no autopsy and/or magnetic resonance imaging [MRI] was performed to suggest direct cardiac involvement by the virus.
Conclusions
COVID-19 patients may be tachycardic in the setting of systemic disease (fever, shortness of breath, pain, etc.). They may also develop rhythm abnormalities in the setting of electrolyte imbalance, and prolonged QTc. 14 In the two critically ill patients previously described, non-sustained monomorphic ventricular tachycardia was the most common arrhythmia. However, interestingly, each patient had three completely different types of arrhythmia during the course of their illness, which are not usually seen simultaneously. Due to myocardial injury induced by SARS-CoV-2, COVID-19 is therefore suspected to potentially lead to a variety of arrhythmias, ranging from asymptomatic- to life-threatening states. These arrhythmias most likely occur more frequently in critically ill patients needing intensive care treatment.
Acknowledgements
The authors are grateful to our colleagues, who contributed invaluable clinical information.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None declared.
Consent
The author(s) confirm that written consent was obtained from patients whose identifiable information was used according to COPE Guidelines.
References
- COVID-19 Map. In: Johns Hopkins Coronavirus Resource Center [Internet]. [cited 9 Apr 2020]. Reterived from: https://coronavirus.jhu.edu/map.html
- Musa S. Hepatic and gastrointestinal involvement in coronavirus disease 2019 (COVID-19): What do we know till now? Arb J Gastroenterol. 2020; 21, 3–8.
- Babapoor-Farrokhran S, Gill D, Walker A, et al. Myocardial injury and COVID-19: Possible mechanisms. Life Sci. 2020; 253, 117723.
- Alhogbani T. Acute myocarditis associated with novel Middle east respiratory syndrome coronavirus. Ann Saud Med. 2016; 36, 78–80.
- Oudit G. Y. et al. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur J Clin Investn. 2009; 39, 618–625.
- Bhatla A, Mayer MM, Adusumalli S. et al. COVID-19 and cardiac arrhythmias. Heart Rhythm. 2020; 17, 1439–1444.
- Schnaubelt S, Breyer MK, Siller-Matula J. et al. Atrial fibrillation: a risk factor for unfavourable outcome in COVID-19? A case report. Europ Heart J - Case Reports 2020 ; 166.
- Babapoor-Farrokhran S, Batnyam U, Wiener PC, et al. Atrioventricular and Sinus Node Dysfunction in Stable COVID-19 Patients. SN Compr. Clin. Med. 2020.
- Babapoor-Farrokhran S, Rasekhi RT, Gill D., et al. Arrhythmia in COVID-19. SN Comp Clin Med. 2020; 1–6.
- Liu K, Feng YY, Deng Y. et al. Clinical characteristics of novel coronavirus cases in tertiary hospitals in Hubei Province. Chin Med J. 2020; 133, 1025–1031.
- Huang C, Wang Y, Li X. et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. 2020; 395, 497–506.
- Kochi AN, Tagliari AP, Forleo GB, et al. Cardiac and arrhythmic complications in patients with COVID-19. J Cardiovasc Electrophysiol. 2020; 31, 1003–1008.
- Goodman S, Weiss Y. & Weissman C. Update on cardiac arrhythmias in the ICU. Curr Opin Critic care. 2008; 14, 549–554.
- Babapoor-Farrokhran S, Alzubi J, Khraisha O. et al. Cardiac Arrhythmias in the Era of COVID-19 Pandemic. Trends Cardiovasc Med. 2020. Reterived from: https://doi.org/10.1016/j.tcm.2020.09.001.



